REVIEW ARTICLE
FRANCO, Guilherme Sousa Leal [1]
FRANCO, Guilherme Sousa Leal. Acute renal failure in the intensive care unit: Diagnosis in critically injured patients. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 11, Vol. 01, pp. 42-53. November 2020. ISSN: 2448-0959, Access Link: https://www.nucleodoconhecimento.com.br/health/critically-injured-patients
SUMMARY
Acute kidney injury (AKI) is a common clinical syndrome in critically ill patients and is associated with longer hospital stay, increased use of hospital resources, and higher morbidity and mortality. The general objective is to present the current definitions of Acute Kidney Injury, profile of the patient with this diagnosis in intensive care units and diagnostic methods with emphasis on the new methods based on the dosage of biomarkers. For the preparation of the review article, the varied scientific publications were consulted in the databases of ScienceDirect, UptoDate, Medline/PubMed, Lilacs and SciELO, using the terms Acute Kidney Injury (AKI), risk factors, urinary biomarkers, nephropathy, UTI (Intensive care unit), renal failure and intensive therapy. The results show that objective-guided therapy in the perioperative period is associated with the reduction of complications and the time of hospitalization. Thus, TGO (glutamic oxalacetic transaminase) can provide beneficial effects, preventing the difficulties of admission to intensive care units
Keywords: Acute Kidney Injury, biomarkers, nephropathy, renal failure, intensive care.
1. INTRODUCTION
Acute kidney injury (AKI) is a common clinical syndrome associated with prolonged hospitalization and increased use of hospital resources, and higher morbidity and mortality (RONCO et al., 2013). It is a complex disorder that occurs in a variety of environments with clinical manifestations ranging from a minimal elevation of serum creatinine to anuric renal failure (MEHTA et al., 2007)
In recent years, although significant research has been devoted to the diagnosis, prevention and treatment of AKI, the incidence and mortality secondary to acute renal dysfunction have remained high (BELLOMO et al., 2004). This clinical condition can occur in any hospital environment with incidence rates ranging from 1% to 25% in critically injured patients. Other studies report (CHERTOW et al., 1998; BY MENDONÇA et al., 2000) prevalence of up to 5% to 7% of all hospitalized patients and two-thirds of critically ill patients (NIGWEKAR; WAIKAR, 2011), depending on the study population and the criteria used to define its presence.
Also in the last decade, the old term “acute renal failure” has been replaced by standardized criteria of Acute Kidney Injury, incorporating small changes in creatinine and urine production, such as the Acute Kidney Injury Network (AKIN) (SAWHNEY; FRASER, 2017). Acute kidney injury (AKI) occurs in up to 50% of patients in the post-surgical intensive care unit, with reported mortality rates of 15% to 80%, with more than 50% of cases being secondary to sepsis (COCA et al 2009; MAXWELL; BELL, 2017).
The general objective of the article is to conduct a systematic literature review addressing the current definitions of Acute Kidney Injury, the profile of the patient with this diagnosis in intensive care units, and diagnostic methods with emphasis on the new models based on the dosage of biomarkers.
2. MATERIAL AND METHODS
This study consists of a literature review, conducted between February and October 2018, based on the analysis of scientific publications, present on the Internet, on data from ScienceDirect, UptoDate, Medline/PubMed, Lilacs, SciELO, especially with the terms Acute Kidney Injury (AKI), Risk Factors, Biomarkers, Nephropathy, ICU, acute renal failure and Intensive Care.
According to the criteria, scientific articles describing the profile of severe patients with acute renal failure in the context of intensive care units were included in this study. Several modalities of studies between 1980 and 2018 were reviewed, including large randomized and multicenter prospective studies, meta-analyses, retrospective observational studies and literary reviews.
3. Review
3.1 ACUTE RENAL FAILURE: DEFINITION.
Acute renal failure (ARF), currently called Acute Kidney Injury (AKI), differs from AKI, mainly because it is a clinical syndrome characterized by the decline in renal function that occurs in a short period of time (Table 1). It is a relatively common complication in critically ill patients and is associated with high morbidity and mortality (NOGUEIRA; OLIVEIRA, 2007).
TABLE 01: Main differences between acute renal failure and chronic renal failure
| Dysfunction/ Parameter | ARF | IRC |
| Story | Days/weeks | Months/Years |
| Hemoglobin | Normal | Reduced |
| Renal “Mass” | Normal | Reduced |
| Osteoarthritis | Out | Present |
| Peripheral Neuropathy | Out | Present |
| Serum Creatinine | Reversibly Increased | Irreversibly Increased |
Source: NOGUEIRA, OLIVEIRA (2007)
Until recently, there was no agreement in the definition of acute kidney injury or a clear consensus on how to prevent or manage this disease. Many models were unable to precisely define the choice of appropriate physiological and clinical parameters for trials of new treatments. The time, intensity and modality of renal replacement therapy, the possible use of extracorporeal blood purification treatment for extra renal diseases and the interaction between renal dysfunction and abnormalities in other organs are factors that should be included among the initiatives to standardize the management of this syndrome (RONCO et al., 2013).
3.2 ACUTE RENAL FAILURE: DIAGNOSIS AND STAGING
It is difficult to ensure an early diagnosis of acute renal vasoconstriction even before tubular dysfunctions occur. In the past, the diagnosis was based on the response of plasma urea levels of the patient with suspected ARF– if levels decreased with intravenous hydration it would be an indication of reversible vasoconstriction (MILLER et al., 1978).
The problem of this approach is that many patients entered a picture of pulmonary congestion, hypoxia and the use of early mechanical ventilation (SCHRIER et al., 2004). From this point, the focus of greater attention in diagnostic investigation became the detailed analysis of urinary sediments (DE MENDONÇA et al., 2000).
Regardless of research, a detailed and accurate history is crucial to diagnose acute kidney injury (ARF) and determine treatment. Distinguishing Acute Kidney Injury from chronic kidney disease is important, but making the distinction can be difficult. In cases of unclarified ARF or in the presence of hematic cylinders renal biopsy (MADAIO et al., 1990) – except in the presence of some situations, among them the most common is active urinary infection (MOTA, 2005).
Biopsy does not replace good clinical examination and anamnesis, but offers a possibility of histopathological investigation and description with details of the renal microstructure. Another consideration is the differentiation between AKI and DRC (Chronic kidney disease)(YANG et al., 2014)
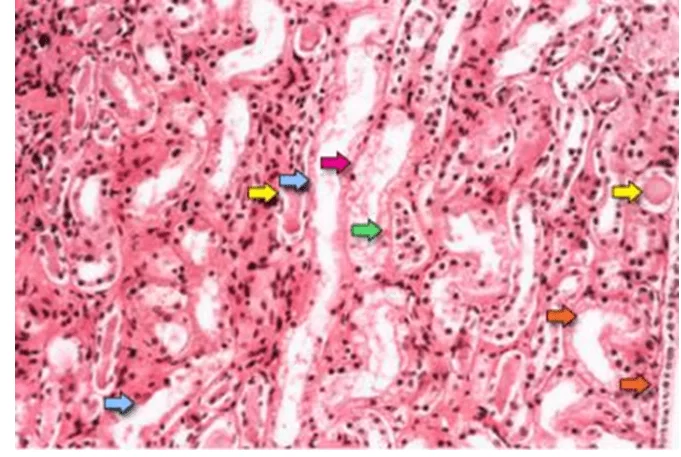
Figure 01 shows characteristics suggestive of acute tubular necrosis that are the diffuse denudation of renal tubular cells with loss of brush edge (blue arrows); flattening of the tubular cells due to tubular dilation (orange arrows); formation of intratubular cluster (yellow arrows); and desoking of cells, which is responsible for the formation of granular molds (red arrow). Finally, intratubular obstruction due to bare epithelium and cellular debris is evident (green arrow). Note that delayered tubular epithelial cells coalesce due to the rearrangement of intercellular reaction molecules (TAN et al., 2009).
FIGURE 01: Photomicrograph of a renal biopsy sample shows the renal medullary region, which is composed mainly of renal tubules.
In an attempt to standardize the definition and classification of AKI, some criteria were created, such as Risk, Injury, Failure, Loss, End-Stage (RIFLE) and Acute Kidney Injury Network (AKIN). Nowadays, these criteria are widely used to classify AKI as a function of serum creatinine and diuresis.
The prospective cohort study using medical records of 190 patients admitted to the Intensive Care Unit found that both the RIFLE score and the AKIN and KDIGO, in a similar way, are good predictors of mortality in patients who are under severe condition. (LEVI et al., 2013).
GRAPH 01: ROC curve and discriminatory capacity for death in critically injured patients according to RIFLE, AKIN and KDIGO
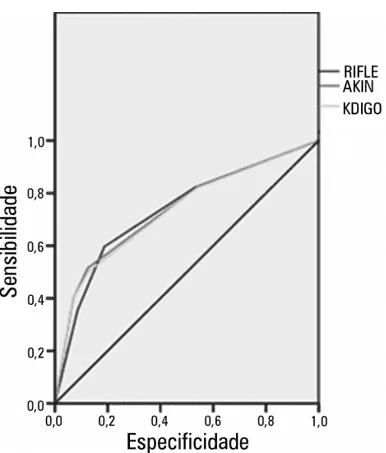
Source: LEVI etal. (2010)
As shown in Graph 01, the authors found that the area on the ROC curve (AUROC) calculated was 0.735 for the RIFLE criterion, 0.74 for The AKIN and 0.733 for The KDIGO, showing the p<0.0001 value for the discrimination of death of the three criteria (LEVI et al., 2010).
3.3 NEW MARKERS IN THE DIAGNOSIS OF ACUTE KIDNEY INJURY
Serum creatinine, which is the most widely used laboratory test for the diagnosis of AKI, is an imperfect marker, since its levels reflect late functional consequences of the lesion rather than direct cell injury, and are not sensitive and specific in the early diagnosis of ARF (NADKARNI et al., 2017).
The development of new biomarkers for early diagnosis, risk stratification and prognosis of acute kidney injury (AKI) is one of the top priorities in worldwide renal research (PARIKH et al., 2014). Some of the most promising substances include lipokalin associated with plasma neutrophil gelatinase (NGAL) (MISHRA et al., 2005), renal injury molecule-1 (KIM-1), (HAN et al., 2002; ICHIMURA et al., 1998), IL-18 (MELNIKOV et al., 2001; PARIKH et al., 2004), cystatin C (HERGET-ROSENTHAL et al., 2004), liver fatty acid binding protein (L-FABP) (KANG et al., 2002; YOSHINO et al., 2003), IL-6 (D’AMICO; BAZZI, 2003), α / π Glutathione S-transferase (GST) (HARRISON et al., 1989; SUNDBERG et al., 1994), and N-acetyl-β-d-glycosaminidase (NAG).
Several studies have shown the efficacy of urinary biomarkers, interleukin-18 (IL-18); lipokalin correlated with plasma neutrophil gelatinase (NGAL); renal injury molecule-1 (KIM-1) and liver-type fatty acid binding protein (L-FABP) to detect AKI before the change in serum creatinine (HO et al., 2015; LIN et al., 2015; NADKARNI et al., 2017). Some of the most promising include lipokalin associated with plasma neutrophil gelatinase (NGAL), renal injury molecule-1 (KIM-1), IL-18,23,24 cystatin C, liver fatty acid binding protein (L-FABP), IL-6.28 α / π S-transferase glutathione (GST), and N-acetyl-β-d-glycosaminidase (NAG).
In general, the apparent diagnostic performance of a biomarker depends not only on its ability to detect lesions, but also on the prevalence of the disease and the sensitivity and specificity of the imperfect gold standard (LASSNIGG et al., 2004).
Some results highlight the importance of prospective and systematic tests of sample storage conditions at biomarker levels prior to the adoption of these tests in clinical practice or research (PARIKH et al., 2014), since urine storage conditions may interfere with the reading of the results obtained.
Nadkarni et al., (2017) concluded that several factors available through urine reagent tape testing are associated with increased concentrations of urinary biomarkers that are independent of clinical kidney injury. Further studies seem to be necessary to evaluate the interference of common urine elements in the levels of biomarkers of interest in exploring the impact of accounting of these elements on their prognostic/diagnostic performance.
Ho et al., (2015), concluded through meta-analysis of 28 studies that reported intraoperative and / or early postoperative measurement of biomarkers in urine (n = 23 studies) or plasma or serum (n = 12 studies). It was concluded that in adults, known biomarkers of urine, plasma and AKI serum have modest discrimination at best when measured within 24 hours after cardiac surgeries, the same studies point out that these biomarkers are still available only for research not being made available in the clinical practice of large centers.
3.4 IDENTIFYING THE CAUSE OF THE ARF
In our study, no tests were identified that compared the accuracy of the urine test with other diagnostic methods such as biopsy and imaging. However, according to Bell and Maxwell (2017), in a meta-analysis conducted by the National Clinical Guideline Centre in 2013, in which 126 publications on prevention, diagnosis and management of acute kidney injury in severe patients were collected, it was strongly recommended that the urine test be conducted with research of blood, protein, leukocytes, nitrites and glucose samples in all patients as soon as acute kidney injury is suspected or detectable. It is assumed that the finding of hematuria and proteinuria in the urine reagent tape test in a patient with AKI indicates probable acute glomerular disease or glomerulonephritis (UCHINO et al., 2004).
In a prospective, multicenter and multinational epidemiological study by Uchino et al., (2004), severe sepsis / septic shock (43.8%), major surgery (39.1%), low cardiac output (29.7) and hypovolemia (28.2%) were the most common conditions associated with the development of acute renal failure in critically injured patients.
Such drugs have been shown, at least in animal models of AKI, reduction of oxygen consumption and metabolic demands of injured renal tubular cells, thus limiting the ischemic damage of external medullary tubular segments (HEYMAN et al., 1994).
3.5. THERAPEUTIC APPROACHES IN THE MANAGEMENT OF ARF
In an acute treatment setting, diuretics are often prescribed to maintain or increase urine production in patients with Acute Kidney Injury (AKI). The reason behind giving diuretics is that they can protect the kidney from ischemic injury while maintaining a non-oliguric state (KARAJALA et al., 2009).
A number of diuretics with diuretic variable pharmacological properties (furosemide, bumetanida, torsemide), thiazide diuretics (metolazone, hydrochlorothiazide, often administered in combination with loop diuretics and diuretics such as mannitol) have been studied and are administered in the AKI setting (KARAJALA et al., 2009). Loop diuretics are the most potent diuretics and remain the most indicated to manage AKI patients (NIGWEKAR; WAIKAR, 2011). AKI management aims to prevent and avoid persistent damage such as nephrotoxic drugs and hypotension, which would decrease TFG (glomerular filtration rate) and local oxygenation.
4. FINAL CONSIDERATIONS
In short, it is known that AKI is a syndrome triggered by a wide range of comorbidities and risk situations. The severely hospitalized patient in an intensive care unit still seems to be the most benefited from the possibility of early diagnosis and correct management of Acute Kidney Injury.
Despite advances in clinical and laboratory research, it is still perceived that its diagnosis is tied to creatinine, urea and the patient’s diuresis record. These measures seem to be effective based on the assumption that they help in increasing the survival of the severe patient.
More clinical studies need to be conducted, as well as meta-analysis studies are of paramount importance for the identification and organization of the large number of laboratory studies involving the collection and dosage of urinary and plasma biomarkers altered in the presence of acute renal failure. New protocols on how to conserve urine samples for these tests need to be elaborated. It is emphasized that the Risk, Injury, Failure, Loss, End-Stage (RIFLE) and Acute Kidney Injury Network (AKIN), although widely used with good degree of accuracy are not effective in the early diagnosis of glomerular injury processes.
As a rule, the application of fluids should be carried out like any other type of medication, considering their indications and individual limitations. In this context, perioperative volume replacement with iso-oncotic solutions is understood as an advantageous aspect, even if additional randomized controlled trials are needed in order to contact its relevance to the outcome. Thus, in summary, the development of a rational fluid administration strategy could help the treatment of patients undergoing low-risk surgery who have an insignificant loss of intravascular volume with crystalloid infusion, through the combination between crystalloid and colloid administration, carefully titrated based on hemodynamic measurements.
Finally, the conclusion is that Objective-Guided Therapy in the Perioperative period is associated with reductions in complications and length of hospitalization. Thus, it is possible to achieve the beneficial effects of TGO, in which it prevents admission to intensive care units.
5. REFERENCES
BELLOMO, R. et al. Open Access Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care, v. 4 n, 8, p. 204–212, 2004.
CHERTOW, G. et al. Independent association between acute renal failure and mortality following cardiac surgery. American Journal of Medicine, v. 104, n. 4, p. 343–348, 1998.
COCA, S. et al. Long-term Risk of Mortality and Other Adverse Outcomes After Acute Kidney Injury: A Systematic Review and Meta-analysis. American Journal of Kidney Diseases, v. 53, n. 6, p. 961–973, 2009.
D’AMICO, G.; BAZZI, C. Urinary protein and enzyme excretion as markers of tubular damage. Current opinion in nephrology and hypertension, v. 12, n. 6, p. 639–43, 2003.
DE MENDONÇA, A. et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Medicine, v. 26, n. 7, p. 915–921, 2000.
HAN, W. et al. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney International, v. 62, n. 1, p. 237–244, 2002.
HARRISON, D. et al. Distribution of glutathione S-transferase isoenzymes in human kidney: basis for possible markers of renal injury. Journal of clinical pathology, v. 42, n. 6, p. 624–8, 1989.
HERGET-ROSENTHAL, S. et al. Early detection of acute renal failure by serum cystatin C. Kidney International, v. 66, n. 3, p. 1115–1122, 2004.
HEYMAN, S. N. et al. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney International, v. 45, n. 4, p. 981–985, 1994.
HO, J. et al. Urinary, Plasma, and Serum Biomarkers’ Utility for Predicting Acute Kidney Injury Associated With Cardiac Surgery in Adults: A Meta-analysis. American Journal of Kidney Diseases, v. 66, n. 6, p. 993–1005, 2015.
ICHIMURA, T. et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. The Journal of biological chemistry, v. 273, n. 7, p. 4135–42, 1998.
KANG, D.-H. et al. Role of the microvascular endothelium in progressive renal disease. Journal of the American Society of Nephrology: JASN, v. 13, n. 3, p. 806–16, 2002.
KARAJALA, V. et al. Diuretics in acute kidney injury. Minerva anestesiologica, v. 75, n. 5, p. 251–7, 2009.
LASSNIGG, A. et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Journal of the American Society of Nephrology: JASN, v. 15, n. 6, p. 1597–605, 2004.
LEVI, T. M. et al. Comparação dos critérios RIFLE, AKIN e KDIGO quanto à capacidade de predição de mortalidade em pacientes graves. Revista Brasileira de Terapia Intensiva, v. 25, n. 4, p. 290–296, 2013.
LIN, X. et al. Urine interleukin-18 in prediction of acute kidney injury: a systematic review and meta-analysis. Journal of Nephrology, v. 28, n. 1, p. 7–16, 2015.
MADAIO, M. P. et al. Renal Biopsy. Nephrology Forum. Kidney International, v. 38, p. 529-543, 1990.
MAXWELL, R.; BELL, C. M. Acute Kidney Injury in the Critically Ill. The Surgical clinics of North America, v. 97, n. 6, p. 1399–1418, 2017.
MEHTA, R. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care, v. 11, n. 2, p. R31, 2007.
MELNIKOV, V. et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. The Journal of clinical investigation, v. 107, n. 9, p. 1145–52, 2001.
MILLER, T. et al. Urinary diagnostic indices in acute renal failure: a prospective study. Annals of internal medicine, v. 89, n. 1, p. 47–50, 1978.
MISHRA, J. et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet, London, England, v. 365, n. 9466, p. 1231–8, 2005.
MOTA, P. C. Indications for renal biopsy. Acta Médica Portuguesa, v. 18, n. 2, p. 147–51, 2005.
NADKARNI, G. et al. Urinalysis findings and urinary kidney injury biomarker concentrations. BMC Nephrology, v. 18, n. 1, p. 218, 2017.
NIGWEKAR, S.; WAIKAR, S. Diuretics in Acute Kidney Injury. Seminars in Nephrology, v. 31, n. 6, p. 523–534, 2011.
NOGUEIRA, C. S.; OLIVEIRA, C. R. Degrandi. Disfunção renal: definição e diagnóstico. Medicina perioperatória, Rio de Janeiro, cap. 64, p. 571-577, 2007.
PARIKH, C. et al. Urinary interleukin-18 is a marker of human acute tubular necrosis. American journal of kidney diseases : the official journal of the National Kidney Foundation, v. 43, n. 3, p. 405–14, 2004.
PARIKH, C. et al. Urine Stability Studies for Novel Biomarkers of Acute Kidney Injury. American Journal of Kidney Diseases, v. 63, n. 4, p. 567–572, 2014.
RONCO, C. et al. Acute Dialysis Quality Initiative (ADQI). Contributions to nephrology. v. 182, p.1–4, 2013.
SAWHNEY, S.; FRASER, S. D. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Advances in chronic kidney disease, v. 24, n. 4, p. 194–204, 2017.
SCHRIER, R. et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. The Journal of clinical investigation, v. 114, n. 1, p. 5–14, 2004.
SUNDBERG, A. et al. Urinary π-Class Glutathione Transferase as an Indicator of Tubular Damage in the Human Kidney. Nephron, v. 67, n. 3, p. 308–316, 1994.
TAN, J. et al. Glomerular function, structure, and number in renal allografts from older deceased donors. Journal of the American Society of Nephrology : JASN, v. 20, n. 1, p. 181–8, 2009.
UCHINO, S. et al. Diuretics and mortality in acute renal failure. Critical Care Medicine, v. 32, n. 8, p. 1669–1677, 2004.
YANG, B. et al. Intravascular Administration of Mannitol for Acute Kidney Injury Prevention: A Systematic Review and Meta-Analysis. (B. Bussolati, Org.)PLoS ONE, v. 9, n. 1, p. e85029, 2014.
YOSHINO, J. et al. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. Journal of the American Society of Nephrology : JASN, v. 14, n. 12, p. 3090–101, 2003.
[1] Doctor. Post graduated in Intensive Care Medicine for Adults.
Submitted: October, 2020.
Approved: November, 2020.















