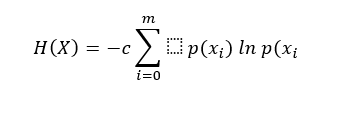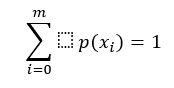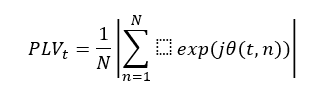ORIGINAL ARTICLE
LUCENA, Marília Marinho de [1], OLIVEIRA, Igor Tchaikovsky Mello de [2], ALBUQUERQUE, Jenifer Emídio de Almeida [3], SANTOS, Wellington Pinheiro dos [4], COSTA, Belmira Lara da Silveira Andrade da [5], RODRIGUES, Marcelo Cairrão Araújo [6]
LUCENA, Marília Marinho de. et al. Non-periodic acoustic stimulation: preliminar reports of an alternative therapeutic for epilepsy. Multidisciplinary Scientific Journal Nucleus of Knowledge. Year. 08, Ed. 06, Vol. 05, pp. 98-114. June 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/alternative-therapeutic, DOI: 10.32749/nucleodoconhecimento.com.br/health/alternative-therapeutic
ABSTRACT
Being refractory is a significant challenge for one third of patients with epilepsy, and there is a need for new treatments. Non-periodic Stimulation (NPS) has shown promise in animal models, but it involves invasive technology. Consequently, non-invasive non-periodic Acoustic Non-periodic Stimulation (ANPS) has been developed based on NPS, with the hope that it could offer anticonvulsant effects. However, it is essential to ensure the safety of ANPS, particularly in individuals with refractory epilepsy, before it can be used in a specific application. To this end, this study aimed to assess the safety of ANPS in patients with refractory epilepsy (n=14; 8 women; 18-49 years old) using Electroencephalographic (EEG) recording and side effect evaluations over a period of five days. A questionnaire was administered immediately following the ANPS exposure and 30 days later. The study also aimed to investigate whether ANPS could trigger electrographic seizure events by counting epileptiform interictal spikes and sharp waves before and after ANPS. Additionally, the effects of ANPS on overall EEG signal properties and synchronicity were studied by calculating entropy and Phase Lock Value (PLV). Results indicate that ANPS did not trigger seizures or side effects after acute exposure or 30 days later. Rather, an improvement in quality of life and a reduction of interictal peaks and sharp waves in the EEG were observed, indicating possible anticonvulsant effects of ANPS. ANPS also increased EEG signal entropy and induced changes in brain synchrony, as evidenced by increased PLV values in some neural networks and decreased PLV values in others. Overall, these findings suggest that ANPS is well-tolerated and safe in patients with refractory epilepsy and may have anticonvulsant properties. Further investigation of its effects on neurophysiology and refractory epilepsy is warranted.
Keywords: Acoustic Stimulation, EEG, Non-Periodic Stimulation, Refractory Epilepsy.
1. INTRODUCTION
Epilepsy is a chronic neurological disorder characterized by recurrent, spontaneous episodes of aberrant synchronicity of neural activity affecting approximately 60 in every 100,000 people (FIEST et al., 2017), corresponding to about 5 million patients worldwide. Among these patients, one third (KWAN et al., 2000; LEE et al., 2012) (1.6 million individuals) is considered refractory, that is, non-responsive to any pharmacological treatment. This condition severely compromises activities of daily living, leisure, and work in epileptic patients (MURRAY, 1993; BORGES et al., 2000; LITT et al., 2001).
Given that a significant number of people continue to experience seizures despite taking medication, there is a strong demand for new methods and concepts to control refractory epilepsy beyond the molecular level (LARIVIÈRE et al., 2021). It is time to shift from solely administering pharmacological agonists, antagonists, release enhancers, or blockers targeting single receptors or neurotransmitter systems towards interfering with the circuital and network levels (SCHMIDT et al., 2021; PEGG et al., 2021; SHIH et al., 2021). This would recruit not only glutamatergic or GABAergic neurons, but also networks with both (and other) neurotransmitters and cell types (e.g., glia, GRIGOROVSKY et al., 2020) that are orchestrated to take brain functional couplings to a different working state, far from the epileptic strong attractors (IASEMIDIS, 1996). These concepts are based on the complexity of neuronal networks, the interchangeability between temporally connected hubs, information distribution, and local recruitments (GARCIA-CAIRASCO, 2009a; GARCIA-CAIRASCO, 2009b). Stimulating the nervous system with different physical protocols, such as electrical pulses of current or voltage, magnetic fields, or even by optical means, is one way to modulate circuital recruitment (for a review, see LEWIS et al., 2016). Among the myriad of methods, non-periodic stimulation (NPS) is a form of electrical stimulation that has been developed in experimental models to specifically interfere with the synchronicity of neural circuits (COTA et al., 2019). In animal models, NPS applied to the basolateral amygdala has been shown to be anticonvulsant against acute (COTA et al., 2009, MEDEIROS et al., 2012; OLIVEIRA et al., 2019) and chronic (OLIVEIRA et al., 2014) seizures. Recently, an independent group has demonstrated that a similar approach also impairs epileptogenesis in a kindling animal model (SANTOS-VALENCIA et al., 2019). The mechanism of action of NPS is by desynchronizing or impairing aberrant levels of synchronization directly in the temporal lobe epileptogenic circuitry (MESQUITA et al., 2011) or indirectly via nucleus accumbens (OLIVEIRA et al., 2019). While promising, NPS faces a significant drawback deriving from the fact that it is invasive and electrodes must be surgically implanted into the brain parenchyma, which hampers general clinical application to epilepsy patients.
In this study, we introduce Acoustic Non-periodic Stimulation (ANPS), a new form of stereo sound that may represent a non-invasive neurostimulation approach based on the same rationale used to develop NPS. ANPS is a stereo soundtrack with a 400 Hz baseband that is interrupted by very short and slightly faster events called “pulses”. It is evident that ANPS was developed for anticonvulsant and antiepileptic purposes. However, the first step is to test the safety of ANPS with refractory epileptic patients, and this is the objective of the present work. In line with this, we also intend to analyze the Electroencephalographic (EEG) of epileptic patients before and after hearing ANPS. Our hypothesis is that the application of ANPS is safe for patients with drug-refractory epilepsy, with no negative impact on the quality of life, no induction of seizures, and no increase in the number of EEG interictal spikes and sharp waves (SSW). We also believe that ANPS would increase the entropy of the EEG signals and change brain synchronicity.
2. METHODS
2.1 ACOUSTIC NON-PERIODIC STIMULATION
ANPS has been developed (patent pending process BR 10 2019 009701 9). The ANPS was constructed as follows: first, a 60000 points x axis was created in the Octave software. This corresponds to 60 epochs of 1000 points each, that is, 60 s of 1 second epoch (divided in 1000 ms). Based on this x axis, a 400 Hz sinusoid was constructed. Four aleatory points (called “pulse locations”) were chosen every second (that is, every 1000 points of the x axis). This implies that ANPS is a 4Hz stimulation (four pulses every second). In each pulse location, 5 points (corresponding to 5 ms) of the signal were substituted for the same number of points of another sinusoid, a 420 Hz with a 20% increase in amplitude compared to the initial 400 Hz one. The soundtrack was later exported to the Audacity 2.0 software (open source, multiplatform audio editor) and recorded in wav format to be reproduced on acoustic stimulus reproduction devices (cellphones, computers) without interruption. The acoustic stimulus has the same baseband in both ears, but the pulse locations are different in the acoustic stimulus emitted for the right and left ear. This strategy was also based on the NPS, as it has maximum effectiveness when applied bilaterally and asynchronously (OLIVEIRA et al., 2018). To use this method, it is necessary to use headphones.
2.2 PARTICIPANTS
Refractory PWE (n = 14, 08 women, 35.4 +- 8.1 years) were randomly recruited at the neurology sector of a hospital in Pernambuco State, Brazil. Patients had generalized absence seizures (57.1%), generalized motor seizures (14.3%), or focal aware motor seizures (28.6%). 85% of them were polymedicated. All procedures were approved by the Ethics Committee (approval number 2.419.085). The informed consent was read and signed by patients. The criteria for refractory epilepsy were defined according to the ILAE classification (KWAN et al., 2010).
2.3 QUESTIONNAIRE OF ADVERSE EFFECTS
When dealing with a new treatment, it is also important to verify the occurrence of side effects after acute and chronic exposure. Patients were asked to attend to the Neurology Sector of the Clinical Hospital of the Federal University of Pernambuco for 5 consecutive days (from 9 to 11 a.m.). All patients were asked to answer socio-demographic assessment questionnaires (semi-structured interviews). They listened to the ANPS for 30 min once a day in the hospital. After each hearing section, they were asked to answer a questionnaire to evaluate adverse effects. This consisted of questions in which patients reported how they were feeling, regarding headache, dizziness, nausea, disorientation, mood swings, seizures, or “others”, where they could describe different ones. Answers included 0 (no symptoms), 1 (for mild symptoms), 2 (for moderate symptoms) or 3 (strong symptoms). Thirty days later, patients were asked to return to the hospital for another evaluation of possible adverse effects.
2.4 EEG RECORDING
Patients were instructed to be sleep-deprived the night before and to sleep for 65 minutes in the hospital, with simultaneous EEG recording. This is a standard protocol used routinely in the Neurology Section and aims to increase neuronal excitability for the diagnosis of epilepsy (KOTAGAL; YARDI, 2008). The purpose was to check if ANPS itself could evoke ictal and interictal epileptic events. The amount of SSW events was quantified in the EEG by two independent observers.
The Neuron-SPECTRUM-4 EP digital EEG recorder (Neurosoft) was used for the acquisition of data, at a sampling rate of 500 Hz and bandpass filtered from 0.5 to 35 Hz (40 dB suppressing and 110 dB common mode rejection ratio). Twenty-one leads were used, 2 for linked auricular reference and 19 recording channels positioned according to the 10-20 system. Impedance was checked (lower than 5 KΩ was accepted). Removal of artifacts was done with visual inspection and Independent Component Analysis (ICA) function of the acquisition software (URIGÜEN; GARCIA-ZAPIRAIN, 2008).
Patients sleep deprived were asked to lie down with their eyes closed in a quiet and shadowed room with a controlled temperature (24 ºC) and the EEG recording started. For ANPS reproduction, all subjects were using headphones. The sleep onset followed international guidelines and the experimenter waited until characteristic changes in the EEG indicative of sleep phase 1, decrease in beta and increase in alpha, was clearly seen and persisted for 5 min (WOLPERT, 1969; HORI. et al., 1968). The initial 20 minutes were called “before acoustic stimulation” (BS) or baseline and no sound was applied. Then, the ANPS started, and there was a ramp increase in the stimulation intensity to reach half the maximum volume of earphones (50-60 dB, a safe range (WHO, 2015) in five minutes. This ensured that all patients could perceive the presence of acoustic stimulation, avoiding very intense stimulation. This procedure did not awake the patients. Then, another 20 minutes of EEG recording were performed simultaneously with the application of ANPS (stimulation period, S). After the end of the ANPS stimulation, this period was called “after stimulation” (AS, 20 min). Only patients who remained in the NREM sleep phase were included in the study. This was verified by visual inspection of the EEG.
2.5 ANALYSIS OF ICTAL AND INTERICTAL EVENTS ON EEG
Epileptic discharges were quantified in BS and AS periods by visual inspection. This was conducted by two blind and independent observers who were previously trained by an experienced clinical neurologist for the correct identification of EEG spikes. The classic format of epileptiform activities was adopted, in which spikes and sharp waves are collectively referred as a single event, called SSW (GOTMAN; GLOOR, 1976). Statistical analysis was performed by comparing the number of SSW between BS and AS. The number of SSW events during BS and AS were normalized to the total value of each patient following the equation ((BS-AS) / BS) * 100, to make possible the comparison of this variable. The normality of the data was verified with the Shapiro-Wilk and Kolmogorov-Smirnov tests. The percentage of SSW events in BS and AS periods was compared using paired t test.
2.6 ENTROPY CALCULATION
One approach to verify if ANPS could change brain electrical activity is the calculation of the entropy of EEG signals. It is well known that many events (COSTA, et al., 2005), including epileptic ones (ARUNKUMAR et al., 2016; MATEOS et al., 2014), generally change EEG entropy. The entropy of EEG was measured to check if ANPS could increase the aleatory firing of cortical neurons. Since ANPS is an acoustic stimulation with random pulses, there is a possibility that it may change the neuronal firing pattern of the acoustic pathway in a bottom-up passage.
Shannon entropy H(X) was calculated as previously described (PHUNG et al., 2014) in all 19 EEG channels using:
Where X are the EEG values (finite discrete variables), X={x1, x2,…xm}, xi∈ R, c is a positive constant, and p(xi) is the probability of xi∈ X satisfying (PHUNG et al., 2014):
The algorithm used was implemented by Malik (2021). Two minutes of EEG recording in the BS and AS periods from all PWE included in the analysis were divided into 120 epochs of 10s each. From these, 30 epochs were randomly chosen for the calculation of entropy. Normality of data was checked with Shapiro-Wilk and Kolmogorov-Smirnov tests. A paired Student’s T test was applied to compare entropy values in BS compared to AS periods.
2.7 PHASE LOCK VALUE (PLV)
The PLV was used to test if ANPS could change the brain synchronicity of EEG recordings. It is well-known that epileptic phenomena are related to an alteration of brain synchronization of electrical activity. The PLV quantifies phase synchrony in scalp local field potential signals of large-scale networks in frequency-specific ranges (LACHAUX et al., 1999). The mathematical algorithm used was created by Namburi (2021), based on Lachaux et al., (1999). EEG data was first filtered using an FIR (finite impulse response). Afterward, a Hilbert transform was performed to quantify the rising and falling of EEG data, and to compute the instantaneous phase (φ), a value between –π and π. The difference between two phase time courses (Δφ) locking between two electrodes of interest was calculated. For every PWE included in this analysis, two electrodes with high SSW spike incidence were chosen. As the PLV algorithm used works with two electrodes for analysis, it was necessary to find some criteria for electrode selection. So, those electrode pairs with the higher SSW spike incidence seemed to us the most appropriate since they probably are showing the most pronounced epileptic phenomenon of the brain. As described (LACHAUX et al., 1999), the PLV at a time t is defined as
where (t, n) is the phase difference 1(t, n) –2 (t, n), and n is the number of trials for each pair of electrodes.
All statistical analyzes were performed with free statistical programs (SÁNCHEZ-VILLENA, 2019) A probability value of p <0.05 was considered significant.
3. RESULTS
3.1 ANALYSIS OF THE ADVERSE EFFECTS OF ANPS IN ADULTS
ANPS was well tolerated by all 14 patients and did not cause seizures or any side effects, either after acute exposure or 30 days later. 40% of patients described themselves as relaxed after hearing ANPS, and 30% described an asleep feeling.
Most patients also stated that there were no adverse effects such as headache, dizziness, nausea, disorientation, mood swings, or seizures. One described nausea, that appeared two days after the start of the acoustic stimulation.
3.2 ANALYSIS OF ICTAL AND INTERICTAL EVENTS ON EEG
No ictal EEG events were induced by the ANPS stimulation. From the initial group of PWE who underwent the whole intervention (n=14), only four (n=04, 02 women, 33+-5 years) of the patients had SSW clearly detectable in the EEG, and those were included in this analysis. All had fronto-temporal focal epilepsy. The AS period was marked by a robust decrease in the average number of EEG SSW when compared to the BS period (Table 1). The average reduction of the SSW number in all patients was 62.4% (Table 1). The average proportion of SSW events in the AS period was 27% for observer 1 and 25% for observer 2, and this reduction was statistically significant for both (p= 0.003 and p=0.005 respectively, paired t-test, one-tailed distribution) (Table 1).
Table 1. Acue effect of ANPS on SSW in 04 patients with refractory epilepsy
| OBSERVER 1 | ||||||
| SSW BS | SSWAS | SSW TOT | PROP BS | PROP AS | TOT | |
| Patient 1 | 443 | 133 | 576 | 0.769097 | 0.230903 | 1 |
| Patient 2 | 932 | 216 | 1148 | 0.811847 | 0.188153 | 1 |
| Patient 3 | 43 | 21 | 64 | 0.671875 | 0.328125 | 1 |
| Patient 4 | 95 | 46 | 141 | 0.673759 | 0.326241 | 1 |
| Average percentages | 0.73 | 0.27* | (p=0.0035) | |||
| OBSERVER 2 | ||||||
| SSW BS | SSW AS | SSW TOT | PROP BS | PROP AS | TOT | |
| Patient 1 | 345 | 101 | 446 | 0.773543 | 0.226457 | 1 |
| Patient 2 | 725 | 111 | 836 | 0.867225 | 0.132775 | 1 |
| Patient 3 | 34 | 15 | 49 | 0.693878 | 0.306122 | 1 |
| Patient 4 | 71 | 35 | 106 | 0.669811 | 0.330189 | 1 |
| Average
Percentages |
0.75 | 0.25* | p=(0.0055) | |||
Source: Authors, 2023. SSW: Spikes and Sharp Waves; BS: Before Sound; AS: After Sound; TOT: total; PROP: propotion; *: p<0.05, Paired t test, one tailed distribution. Data represents the number of SSW before and after sound (BS and AS, respectively). *: p<0.05, Paired t test, one tailed distribution. Normality was checked by the Shapiro-Wilk test.
3.3 ENTROPY AND PLV ANALYSIS
ANPS induced a statistically significant increase in the entropy of EEG signals. The entropy estimation of the BS period was 2.65 ± 0.33 J/K, while the AS was 4.87 ± 0.55 J/K (p=0.008, paired student t-test) (Fig 1A
Figure 1 Entropy and PLV analysis. 1. A. Entropy. The ANPS induced an increase in entropy values of EEG recordings after ANPS *: p<0.05, paired t-test. 1.B.PLV analysis. PLV of the EEG of a representative patient before and after ANPS stimulation
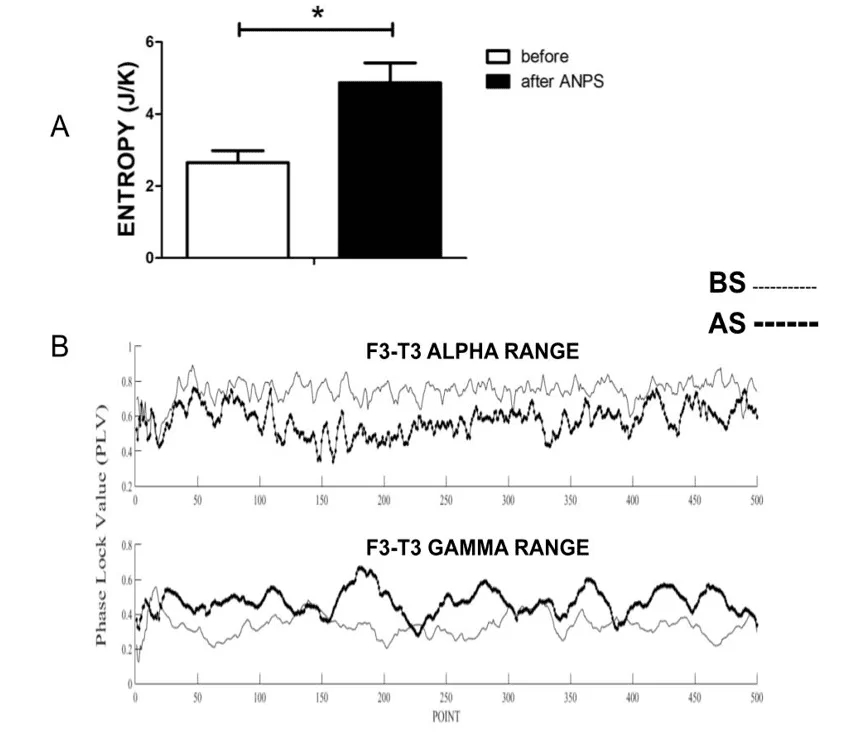
In the analysis of the PLV, the four patients who presented SSW in the EEG were selected. The electrodes selected were those with the highest incidence of SSW. Table 2 contains the PLV results of EEG recordings. AS was seen both with increased or decreased PLV values compared to BS depending on the network analyzed, even in the same person (Fig. 1.B). These results were significantly different (p<0.0001, Wilcoxon signed rank test, two-talied). The PLV analysis showed that some networks had an increase in synchronization, while others a decrease. The effect of sound was not the same in all studied networks.
Table 2. PLV calculation of EEG recordings from patients before (BS) and after (AS) the ANPS. Experiment 2
| atient | Delta theta | Alpha | beta | gamma | EEG chanel | ||||
| RH | LH | RH | LH | RH | LH | RH | LH | RH | |
| Patient 1 | ↑AS | ↑AS | ↑AS | ↑AS | ↓AS | ↑AS | ↓AS | ↑AS | RH F4 C4 |
| Patient 2 | ↓AS | ↓AS | ↓AS | ↓AS | ↑AS | ↑AS | ↑AS | ↑AS | LH F7 T3 |
| Patient 3 | ↑AS | ↑AS | NS | ↑AS | ↓AS | ↑AS | ↓AS | ↑AS | LH F3 e Fz |
| Patinet 4 | ↓AS | ↑AS | NS | ↑AS | ↑AS | ↑AS | NS | ↑AS | LH T3 T5 |
Source: Authors, 2023. BS: before stimulation; AS after stimulation The down arrows indicate that the PLV of the two evaluated EEG channels had a statistically significant decrease compared to the BS period. Up arrows indicate that the AS period had a statistically significant increase in PLV value when compared to the BS period for the two analyzed channels. P was considered significant if < 0.05. RH: right hemisphere; LH: left hemisphere; F4-C4: neuronal network dyad analyzed involving electrodes F4 and C4; F7 T3: dyad F7 and T3; F3 Fz: dyad F3 and Fz; T3 T5: dyad T3 and T5. Mann-Whitney paired test (all data had non-normal behavior, according to Shapiro-Walk analysis of normality)
4. DISCUSSION
The present work is a preliminary pilot study whose main objective was to verify the safety of ANPS for epileptic patients. The results confirmed our hypothesis that ANPS would be safe and change brain oscillatory activity and synchronization. Our intention was to bring translational results to the field of Clinical Neurology in Epilepsy – focusing especially on refractory patients – using rigorously planned and executed protocols developed initially in animal models of epilepsy. This is the first study describing the effect of ANPS in refractory patients with epilepsy.
The clinical protocol occurred with no major difficulties and proved to be feasible. No ictal EEG events or behavioral seizures were induced by ANPS in any of the patients in the present study. ANPS did not induce or increase SSW EEG events, which was verified by two trained and independent observers (Table 1). On the contrary, there was a decrease in the number of SSW events. In this experiment, SSW quantification was performed to rule out possible ictal events and ANPS proconvulsant activity. However, this experiment resulted in a sustained decrease in SSW. Although this is not a solid criterion for establishing an anticonvulsant effect in humans (PILLAIAND, 2006), it has been associated with an improvement in epilepsy in some circumstances (PILLAIAND, 2006; STALEY et al., 2011; LEE et al., 2012). In the present study, it can be guaranteed that all patients remained in non-REM phases due to the short duration (65 min) and visual inspection of the EEG. It is known that the number of SSW events increases as non-REM sleep progresses and decreases only in REM sleep. Further experiments are needed to understand SSW events, the type of epilepsy, and ANPS effect. Even if ANPS by itself cannot induce a sustained anticonvulsant effect, it is still interesting to see whether it could have a synergistic (add-on) effect with other anticonvulsant approaches such as cannabidiol (KAUR et al., 2021; ALVES et al., 2020).
The results of entropy and PLV (Fig. 1 A and B, respectively) clearly confirm our hypothesis that ANPS alters brain function, oscillatory activity, and synchronization. ANPS can increase the entropy of EEG signals. Zones of epilepticus are known to have decreased entropy (ARUNKUMAR et al., 2016), and this has been used as an electrophysiological marker of epileptic brain tissue eligible for surgical resection (VILA-VIDAL et al., 2020). Thus, the increase in entropy observed in the present work may mean that ANPS is driving the brain away from the epileptic functioning mode. In addition, ANPS alters brain synchronicity (see Table 2). As PLV provides a mathematical quantification of phase lock and synchrony, ANPS does not act in the same way in all neural networks. In fact, there is an increase in the synchronization of some networks and frequency bands and a decrease in others (Table 2). As stated by Cota et al., (2016), it seems reasonable that an anticonvulsant effect obtained by manipulating the circuit network could simultaneously induce an increase in synchronization in some networks and a decrease in others. In the present work, this can be clearly seen in PLV measurements. Consequently, an anticonvulsant effect would be more complex than simply making desync ubiquitous across all networks. Rather, it would be related to a reestablishment of a natural and healthy oscillatory profile, synchronized or not, depending on the functionality of the network and the state of the brain. Under anticonvulsant treatment, brain connectivity would change between many dynamic states of physiological coupling, thus avoiding the recruitment of frequent strong attractors for the recruitment of epileptic phenomena. Despite the statistical significance obtained (Table 2), it is not clear so far whether there might be a pattern of PLV changes in relation to specific types of epilepsy (e.g., focal or generalized), and further experiments are needed to establish this with certainty.
Currently, it is not possible to establish the neural mechanisms underlying the effects of ANPS in the brain. As a form of bottom-up stimulation, ANPS likely influenced the cerebral cortex. In this case, as an acoustic stimulus, it may have started in the primary auditory cortex in the temporal lobe and progressed to other cortical areas. We believe that ANPS may have interfered with the synchronization of brainstem nuclei in the ascending auditory neuronal pathways. Neuronal networks located in brainstem nuclei involved in the localization of acoustic sources (for example, superior olivary complex, medial nucleus of the trapezoidal body, among others) could have entered a new functional coupling. This may, in turn, have caused cortical changes in SSW synchronization and suppression. This reasoning is in line with the correlated pathological phenomena of reflex seizures caused by sensory stimulation observed in susceptible humans (WOLF, 2017) and in different rodent species (GARCIA-CAIRASCO et al., 2017). Although humans are much more susceptible to visual stimuli and rodents to acoustic stimuli, they all seem to share mechanisms related to the dysfunctional synchronization of neural oscillation generated in preexisting functional anatomical networks (PARRA et al., 2003; SZÜCS et al., 2019), including those involving midbrain-hindbrain connections (MORAES et al., 2005). Therefore, exogenous desynchronizing stimuli delivered to seizure-prone sensory networks may produce anticonvulsant effects. Recently, it has been hypothesized that the anticonvulsant property of NPS is because its temporal structure – intervals between pulses distributed as a unit-exponent power law – mimics the natural input that most easily causes physiological neural recruitment (COTA et al., 2019). By analogy, once temporally structured in the same way, ANPS can be a natural input source that competes with aberrant oscillations for the recruitment of susceptible circuits while inducing physiological neural activity. In a similar context, it is intriguing to know that a paradoxical effect of acoustically associated cortical and limbic hyperactivity is the phenomenon of tinnitus, a phantom auditory phenomenon (LAUER et al., 2018). In fact, in this case, the lack of auditory information usually associated with lesions in the periphery (e.g., ototoxicity and acoustic trauma) recruits cortical and limbic areas as a compensatory mechanism. On the other hand, an example of this type of interaction can be seen in a study that tests the possibility of interrupting the adverse effects of tinnitus on the daily activities of patients with the application of neurofeedback at alpha-delta frequencies (GÜNTENSPERGER et al., 2019).
Despite centuries of experience with pharmacological treatments for epilepsy, there are many advantages of non-pharmacological neuromodulation techniques. A classic example is vagus nerve stimulation. Drugs act at the molecular and cellular levels, while neuromodulation techniques directly influence the network circuitry, recruiting the molecular and cellular levels from within. This suggests fewer side effects, such as those that impact school learning and systemic organs.
Further studies with a larger number of patients, different types of epilepsy, and the use of high-density EEG with source analysis are needed to understand the effects of ANPS on brain physiology.
5. CONCLUSION
Present results demonstrate that ANPS is safe and well tolerated by refractory epileptic patients and can change brain synchronization and oscillatory activity. Further studies with a greater number of patients and different types of epilepsy are needed to understand the effects of ANPS on brain physiology.
REFERENCES
ALVES, P. et al. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacological research, v. 157, p. 104822, 2020.
ARUNKUMAR, N.; KUMAR, K. R.; VENKATARAMAN, V. Automatic detection of epileptic seizures using new entropy measures. Journal of Medical Imaging and Health Informatics, v. 6, n. 3, p. 724-730, 2016.
BORGES, M. A. et al. Epileptic syndromes and seizures and their relationship with work: a prospective ambulatory study in 412 epileptic patients. Archives of Neuropsychiatry, v. 58, p. 691-697, 2000.COSTA, M.; GOLDBERGER, A. L.; PENG, C.-K. Multiscale entropy analysis of biological signals. Physical review E, v. 71, n. 2, p. 021906, 2005.
COTA, V. R. et al. Distinct patterns of electrical stimulation of the basolateral amygdala influence pentylenetetrazole seizure outcome. Epilepsy & Behavior, v. 14, n. 1, p. 26-31, 2009.
COTA, V. R. et al. Nonperiodic stimulation for the treatment of refractory epilepsy: Applications, mechanisms, and novel insights. Epilepsy & Behavior, v. 121, p. 106609, 2021.
COTA, V. R. et al. The epileptic amygdala: toward the development of a neural prosthesis by temporally coded electrical stimulation. Journal of Neuroscience Research, v. 94, n. 6, p. 463-485, 2016.
MEDEIROS, D. C. et al. Anatomically dependent anticonvulsant properties of temporally-coded electrical stimulation. Epilepsy & Behavior, v. 23, n. 3, p. 294-297, 2012.
OLIVEIRA, J. C. et al. Asynchronous, bilateral, and biphasic temporally unstructured electrical stimulation of amygdalae enhances the suppression of pentylenetetrazole-induced seizures in rats. Epilepsy research, v. 146, p. 1-8, 2018.
OLIVEIRA, J. C. et al. Seizure suppression by asynchronous non-periodic electrical stimulation of the amygdala is partially mediated by indirect desynchronization from nucleus accumbens. Epilepsy Research, v. 154, p. 107-115, 2019.
OLIVEIRA, J. C. et al. Temporally Unstructured Electrical Stimulation to the Amygdala Suppresses Behavioral Chronic Seizures of the Pilocarpine Animal Model. Epilepsy Behav, v. 36, p. 159-164, 2014.
FIEST, K. M. et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology, v. 88, n. 3, p. 296-303, 2017.
GARCIA-CAIRASCO, N. Learning about brain physiology and complexity from the study of the epilepsies. Brazilian Journal of Medical and Biological Research, v. 42, p. 76-86, 2009a.
GARCIA-CAIRASCO, N. Puzzling challenges in contemporary neuroscience: insights from complexity and emergence in epileptogenic circuits. Epilepsy & Behavior, v. 14, n. 1, p. 54-63, 2009b.
GARCIA-CAIRASCO, N.; UMEOKA, E. H. L; OLIVEIRA, J. A. The Wistar Audiogenic Rat (WAR) strain and its contributions to epileptology and related comorbidities: history and perspectives. Epilepsy & Behavior, v. 71, p. 250-273, 2017.
GOTMAN, J.; GLOOR, P. Automatic recognition and quantification of interictal epileptic activity in the human scalp EEG. Electroencephalography and clinical neurophysiology, v. 41, n. 5, p. 513-529, 1976.
GRIGOROVSKY, V.; BRETON, V. L.; BARDAKJIAN, B. L. Glial Modulation of Electrical Rhythms in a Neuroglial Network Model of Epilepsy. IEEE Transactions on Biomedical Engineering, v. 68, n. 7, p. 2076-2087, 2020.
GÜNTENSPERGER, D. et al. Investigating the efficacy of an individualized alpha/delta neurofeedback protocol in the treatment of chronic tinnitus. Neural plasticity, v. 2019, 2019.
IASEMIDIS, L. D.; SACKELLARES, J. C. REVIEW: Chaos Theory and Epilepsy. The Neuroscientist, v. 2, n. 2, p. 118-126, 1996.
KAUR, J. et al. Potential anti-epileptic phytoconstituents: An updated review. Journal of ethnopharmacology, v. 268, p. 113565, 2021.
KOTAGAL, P.; YARDI, N. The relationship between sleep and epilepsy. In: Seminars in pediatric neurology. WB Saunders, 2008. p. 42-49.
KWAN, P. et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. 2010.
KWAN, P.; BRODIE, Martin J. Early identification of refractory epilepsy. New England Journal of Medicine, v. 342, n. 5, p. 314-319, 2000.
LACHAUX, J.‐P. et al. Measuring phase synchrony in brain signals. Human brain mapping, v. 8, n. 4, p. 194-208, 1999.
LARIVIERE, S. et al. Connectome biomarkers of drug‐resistant epilepsy. Epilepsia, v. 62, n. 1, p. 6-24, 2021.
LAUER, A. M. et al. Behavioral animal model of the emotional response to tinnitus and hearing loss. Journal of the Association for Research in Otolaryngology, v. 19, p. 67-81, 2018.
LEE, K. J.; SHON, Y. M.; CHO, C. B. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotactic and functional neurosurgery, v. 90, n. 6, p. 379-385, 2012.
LEWIS, Philip M. et al., Brain neuromodulation techniques: a review. The neuroscientist, v. 22, n. 4, p. 406-421, 2016.
LITT, Brian et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron, v. 30, n. 1, p. 51-64, 2001.
MALIK, J. Multiscale Sample Entropy. Available in: www.mathworks.com/matlabcentral/fileexchange/62706-multiscale-sample-entropy MATLAB Central File Exchange. Accessed November 29, 2021.
MATEOS, D.; DIAZ, J. M.; LAMBERTI, P. W. Permutation entropy applied to the characterization of the clinical evolution of epileptic patients under pharmacological treatment. Entropy, v. 16, n. 11, p. 5668-5676, 2014.
MESQUITA, M. B. S. et al. Distinct temporal patterns of electrical stimulation influence neural recruitment during PTZ infusion: an fMRI study. Progress in biophysics and molecular biology, v. 105, n. 1-2, p. 109-118, 2011.
MORAES, M. F. D. et al. An electrographic analysis of the synchronous discharge patterns of GEPR-9s generalized seizures. Brain research, v. 1046, n. 1-2, p. 1-9, 2005.
MURRAY, J. Coping with the uncertainty of uncontrolled epilepsy. Seizure, v. 2, n. 3, p. 167-178, 1993.
NAMBURI, P “Phase Locking Value website”, https://www.mathworks.com/matlabcentral/fileexchange/31600-phase-locking-value, MATLAB Central File Exchange. Accessed November 29, 2021.
PARRA, J. et al. Gamma‐band phase clustering and photosensitivity: is there an underlying mechanism common to photosensitive epilepsy and visual perception?. Brain, v. 126, n. 5, p. 1164-1172, 2003.
PEGG, E. J. et al. Interictal electroencephalographic functional network topology in drug‐resistant and well‐controlled idiopathic generalized epilepsy. Epilepsia, v. 62, n. 2, p. 492-503, 2021.
PHUNG, D. Q. et al. Using Shannon Entropy as EEG Signal Feature for Fast Person Identification. In: ESANN. 2014. p. 413-418.
PILLAI, J.; SPERLING, M. R. Interictal EEG and the diagnosis of epilepsy. Epilepsia, v. 47, p. 14-22, 2006.
SÁNCHEZ-VILLENA, A. Uso de programas estadísticos libres para el análisis de datos: Jamovi, Jasp y R. Revista perspectiva, v. 20, n. 1, p. 112-114, 2019.
SCHMIDT, Sarah et al. Complex effects of eslicarbazepine on inhibitory micro networks in chronic experimental epilepsy. Epilepsia, v. 62, n. 2, p. 542-556, 2021.
SHIH, Yao-Chia et al. Seizure frequency is associated with effective connectivity of the hippocampal–diencephalic–cingulate in epilepsy with unilateral mesial temporal sclerosis. Brain Connectivity, v. 11, n. 6, p. 457-470, 2021.
HORI, T et al. Proposed supplements and amendments to ‘A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry and clinical neurosciences, v. 55, n. 3, p. 305-310, 2001.
STALEY, K. J.; WHITE, A.; DUDEK, F. E. Interictal spikes: harbingers or causes of epilepsy?. Neuroscience letters, v. 497, n. 3, p. 247-250, 2011.
SZŰCS, A. et al. Reflex seizure triggering: Learning about seizure producing systems. Seizure, v. 69, p. 25-30, 2019.
URIGÜEN, J. A.; GARCIA-ZAPIRAIN, B.. EEG artifact removal—state-of-the-art and guidelines. Journal of neural engineering, v. 12, n. 3, p. 031001, 2015.
VILA-VIDAL, M. et al. Low entropy map of brain oscillatory activity identifies spatially localized events: A new method for automated epilepsy focus prediction. NeuroImage, v. 208, p. 116410, 2020.
WOLF, P. Reflex epileptic mechanisms in humans: lessons about natural ictogenesis. Epilepsy & Behavior, v. 71, p. 118-123, 2017.
WOLPERT, E. A. A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Archives of General Psychiatry, v. 20, n. 2, p. 246-247, 1969.
WORLD HEALTH ORGANIZATION (WHO). Make Listening Safe. World Health Organization; 015. https://apps.who.int/iris/bitstream/handle/10665/177884/WHO_NMH_NVI_15.2_eng.pdf Accessed November 29, 2015.
[1] Master in Neuropsychiatry and Behavioral Sciences – UFPE; Specialists in Multiprofessional Neurosciences and Neurofunctional Physiotherapy; Graduated in Physiotherapy. ORCID: 0000-0002-1731-0860. Currículo Lattes: http://lattes.cnpq.br/7751944803136886.
[2]Doctor and Master in Neuropsychiatry and Behavioral Sciences – UFPE; Specialists in Multiprofessional Neurosciences, graduated in Biomedicine. ORCID: 0000-0001-8327-2308. Currículo Lattes: http://lattes.cnpq.br/7340814219343971.
[3] Master in Biomedical Engineering – UFPE, graduated in Nursing. ORCID: 0009-0007-4564-0019. Currículo Lattes: http://lattes.cnpq.br/6262936771420818.
[4] PhD in Electrical Engineering from UFCG; Master in Electrical Engineering from UFPE; Electronic engineer. ORCID: 0000-0003-2558-6602. Currículo Lattes: http://lattes.cnpq.br/6413917211782026.
[5] PhD in Biological Sciences (Biophysics) by UFRJ; Master in Biological Sciences (Physiology) -UFPE; Degree in Biological Sciences -UFRPE. ORCID: 0000-0002-3567-6436. Currículo Lattes: http://lattes.cnpq.br/3487590330093989.
[6] Doctor and Master in Psychobiology – USP; Graduation in Biological Sciences Medical Modality – UNIFESP. Advisor. ORCID: 0000-0003-3646-6008. Currículo Lattes: http://lattes.cnpq.br/8243956522121701.
Sent: April 17, 2023.
Approved: June 20, 2023.