REVIEW ARTICLE
MANFREDI, Márcia Azevedo Bastian [1], SILVA, Marcos Antonio Segatto [2], MERLINI, Cláudia [3]
MANFREDI, Márcia Azevedo Bastian. SILVA, Marcos Antonio Segatto. MERLINI, Cláudia. The use of electrospinning in the development of systems for buccal drug delivery: a review. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year. 08, Ed. 10, Vol. 02, pp. 100-135. October 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/use-of-electrospinning, DOI: 10.32749/nucleodoconhecimento.com.br/health/use-of-electrospinning
ABSTRACT
Electrospinning is a simple, low cost and versatile technology used for the manufacture of nanomaterials. The electrospun nanofibers produced are characterized by high porosity and large specific surface area, factors that make the membranes potentially useful in the development of systems for drug release. When it comes to drug release in the oral cavity, the nanofibers are highlighted due to the advantage of easy modulation of the drug release profile, by monitoring the morphology, porosity, chemical composition of the fibers and the electrospinning technique. In this sense, this study developed a literature review on the use of the electrospinning technique to obtain buccal drug delivery systems. Scientific articles published in the databases Science Direct and Embase were used, with the following descriptors and their combinations: [electrospinning] and [buccal drug delivery]. Articles published between 2013 and 2023 were considered. For the inclusion of the studies, the following criteria were used: articles published in English, experimental studies, and the descriptors should appear in the title, abstract, or keywords. Fourteen articles were included in the final analysis and from them it was verified the wide use of the electrospinning technique for the development of buccal drug delivery systems and the employment of active substances of various pharmacological classes. The analyzed studies conclude that the use of electro-spinning of drugs associated with other pharmaceutical excipients are promising tools in the development of drug delivery systems in the oral cavity, since they avoid first-pass hepatic metabolism, enzymatic degradation of the drug, present ease of administration and allow easy removal of the system in case of adverse events.
Keywords: Electrospinning, Buccal drug delivery, Drug delivery systems, Controlled drug release, Electrospun fibers.
1. INTRODUCTION
The electrospinning technique has gained great notoriety in recent decades, mainly due to its ability to produce materials consisting of fibers with diameters ranging from nanometers to a few microns. Nanofibers give rise to membranes with three-dimensional porous architecture and present characteristics such as large specific surface area and interconnectivity, which makes them suitable for various biomedical applications, including drug delivery systems (Lannutti, et al., 2007; Alazab et al., 2017; Al-Hazeem, 2018; Zubir et al., 2020).
Electrospinning is a simple technique and the equipment (Figure 1) consists of a high voltage source, an infusion pump with a syringe and a thin metallic needle, with automatic control of the polymeric solution flow and a grounded metallic collector (Frenot; Chronakis, 2003; Sofi et al., 2020).
In the electrospinning process, an electric field is applied to the syringe containing the polymer to be electrospun. the applied electric field, overcoming surface tension and viscosity, ejects the polymer solution and during the journey to the collector, the solvent evaporates and the solid fibers are deposited (Contreras-Cáceres et al., 2019).
Figure 1. Electrospinning process
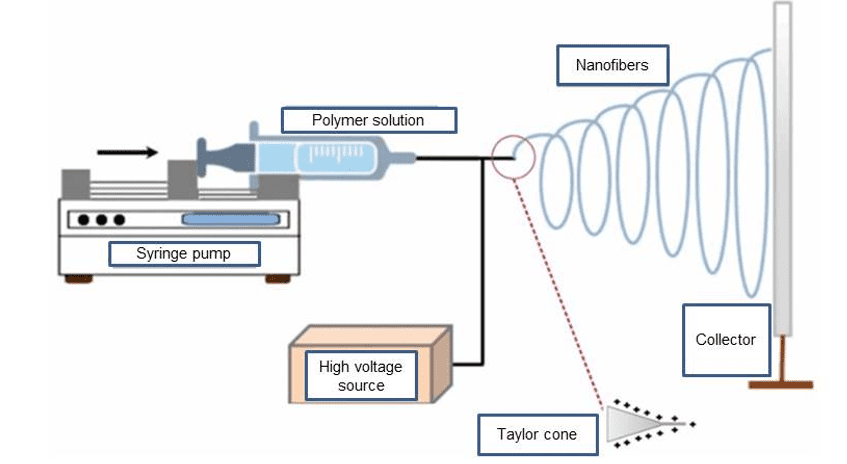
The process depends on several parameters generically classified into solution, process and environmental parameters, which play an important role in the generation of nanofibers and their adjustment allows to obtain fibers with different dimensions and quality (Zubir et al., 2020; Alazab et al., 2017; Lalia et al., 2013).
The solution parameters are: polymer molar mass, solution viscosity, surface tension and solution conductivity. The environmental parameters are the ambient temperature and relative humidity and the process parameters include the voltage, the solution flow rate in the syringe pump, the needle diameter and the distance from the needle tip to the collector (Bhardwaj; Kundu, 2010; Chen et al., 2018b; Rošic et al., 2013).
Electrospun fibers have a high surface area and can be produced in various structures, shapes, and sizes through changes in processing parameters and experimental setup. Controlling the composition of the nanofibers allows the establishment of specific functionalities (Deepak; Goyal; Rath, 2018), and for this purpose they can be manufactured from a variety of polymers (Russo et al., 2016).
The oral administration of drugs can be defined as the administration of drugs in the buccal mucosa, located on the inside of the cheek, inside the mouth, which allows the local and systemic administration of drugs (Alazab et al., 2017). This pathway avoids first-pass metabolism, enzymatic degradation of drugs and facilitates drug administration in individuals with swallowing difficulties (Sofi et al., 2020). Among the existing dosage forms, it is believed that mucoadhesive buccal films are more easily administered due to their flexibility, customizable size and possibility of removal in case of toxicity (Ansari; Sadarani; Majumdar, 2018).
The development of an ideal system for oral drug release is still a great challenge for science. Several characteristics are required for mucosal adhesion, dissolution of the dosage form and pharmacological action of the drug, such as: controlled release or immediate use of the drug; adequate mucosal adhesion strength; oral cavity shear strength and palatability (Palem; Chaitanya; Yamsani, 2011; Carvalho; Chorilli; Gremião 2014).
Electrospun fibers are considered excellent drug carriers and their use in the production of oral active delivery systems has shown a broad future in the production of new pharmaceutical forms (Zubir et al., 2020; Contreras-Cáceres et al., 2019; Hu, et al., 2014; Bhardwaj; Kundu, 2010; Porter; Henson; Popat, 2009; Sill; Von Recum, 2008). Recent studies have shown that electrospun mats can be used for the administration of controlled-release and immediate-release drugs and are an alternative for the release of drugs with low solubility under physiological conditions (Alkahtani et al., 2021; Destefano; Khan; Tabada, 2020).
In this sense, this review aims to evaluate the studies carried out in the last ten years in the research and development of pharmaceutical formulations for the delivery of drugs in the buccal cavity.
2. METHODS
This is a literature review on the use of the electrospinning process to obtain buccal drug delivery systems. This study used scientific articles published in Science Direct and Embase databases, using the descriptors and their combinations: [electrospinning] and [buccal drug delivery]. Articles published between 2013 and 2023 were considered. For inclusion of studies, the following criteria were used: articles published in English, experimental studies and descriptors should be included in the title, abstract or in the keywords. Review articles, authors’ opinions, book chapters, editorials and letters to the editor were excluded from the study.
In the first stage of study selection, the abstracts of all articles obtained were read. Duplicated articles and those that did not use electrospinning as a method of drug delivery system development were excluded or the system developed was not intended to drug release in the oral mucosa. The other articles were read in their entirety, which enabled the organization of the studies according to the following criteria: authors/year, city, purpose of the study, drug used, polymers used, electrospinning technique used and main results.
3. RESULTS AND DISCUSSION
The initial review resulted in 31 studies, 8 being duplicates, totaling 23 articles. Of these, 8 were excluded, 3 studies for not use the electrospinning technique, 4 studies for being a literature review and 1 study for being the development of transdermal drug delivery. Eighteen articles were included in the final analysis, as shown in Figure 2, with the flowchart of the steps of identification, selection and inclusion of articles for the development of this review. According to Table 1, it was found that since 2017, more research on the subject has been published and the use of electrospinning using different techniques associated with other pharmaceutical excipients, were the main results found.
Figure 2. Study identification and selection flowchart
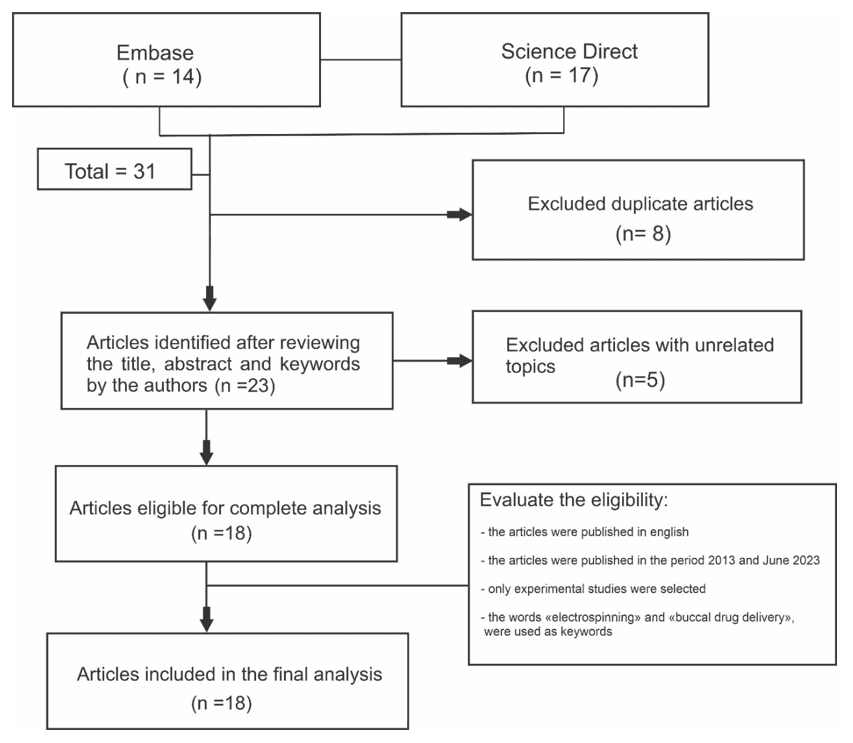
In recent years, studies with fibers based on polymers with large surface area, biocompatibility and biological activity obtained from the electrospinning process have shown enormous relevance in the field of biomedical areas. Research demonstrates that electrospinning is a versatile technique for producing nanofibrous from various types of materials. The large surface-to-volume ratio, porous structure and nanoscale diameter of the fibers provide unique properties to electrospun nanofibrous materials, which allows the incorporation of bioactive aiming at the production of biofunctional materials.
This study highlights the progress in the development of electrospinning techniques emphasizing pharmaceutical application as conventional and new drug delivery systems in buccal cavity. It comprises a series of 18 research articles, which show the great potential of the technique in studies with different methodologies developed by researches from 10 different countries and that contribute significantly to science.
Table 1. Selected studies with development of electrospun systems for drug release into the buccal cavity
| AUTHOR | STUDY LOCATION
|
STUDY PURPOSE | DRUG | USED POLYMERS | ES TECHNICIAN | MAIN RESULTS OF THE STUDY |
| Stie et al.
(2023)
|
DENMARK | THE AIM OF THIS STUDY WAS TO DEVELOP A BIOCOMPATIBLE MULTI-LAYERED DDS FROM HEREON DENOTED NANOFIBER-ON-FOAM-ON-FILM (NFF) FOR OROMUCOSAL DELIVERY OF THERAPEUTIC PEPTIDES
|
THERAPEUTIC PEPTIDES DESMOPRESSIN | CHITOCEUTICALS CHITOSAN
POLYETHYLENE OXIDE |
ELECTROSPINNIG
20 KV 15 CM |
A NOVEL DRUG DELIVERY SYSTEM WAS DEVELOPED FROM MUCOADHESIVE NANOFIBERS IN FREEZE-DRIED FOAM FOR RELEASE OF THERAPEUTIC PEPTIDES. THE STUDY EVALUATED THE MORPHOLOGICAL, MECHANICAL AND MUCOADHESIVE PROPERTIES OF THE SYSTEM AND THE RELEASE AND PERMEATION OF DESMOPRESSIN THROUGH PORCINE ORAL MUCOSA EX VIVO. THE SYSTEM WAS FOUND TO BE HIGHLY SUITABLE FOR ORAL DRUG DELIVERY. |
| Friedl et al.
(2022)
|
AUSTRIA | THE AIM OF THIS STUDY WAS THEREFORE TO COMBINE THE ADVANTAGES OF ELECTROSPUN FIBERS, MUCOADHESIVE THIOLATED POLYMERS AND SEDDS FOR DEVELOPMENT OF MUCOADHESIVE ELECTROSPUN SEDDS-LOADED PATCHES.
|
CURCUMIN | MUCOADHESIVE THIOLATED POLYMERS AND SEDDS, POLYVINYL PYRROLIDONE (PVP) | ELECTROSPINNING
12-13 KV 6ML/H |
THE DRUG DELIVERY SYSTEM WAS DEVELOPED FROM LIQUID SELF-EMULSIFYING DRUG DELIVERY SYSTEMS (SEDDS), SOLID BUCCAL ADHESIVE SYSTEMS AND CURCUMIN. SEDDS LOADED WITH THE DRUG WERE ADDED TO A POLYMERIC ELECTROPHATING SOLUTION CONTAINING PLGA AND PVP AND IN PARALLEL WAS ELECTROPHATED THIOLATED POLYACRYLIC ACID SOLUTION TO INCREASE MUCOADHESION. THE SYSTEM SHOWED PROMISE FOR BUCCAL DRUG DELIVERY ESPECIALLY FOR WATER INSOLUBLE DRUGS. |
| Zhao et al.
(2022) |
CHINA | IN THIS INVESTIGATION, AN ATTEMPT HAS BEEN MADE TO UTILIZE CM-CD, WHICH WATER SOLUBILITY GREATLY IMPROVED AND A CHEAP POLYSACCHARIDE EXCIPIENT FOR PRODUCING BUCCAL FILM CONTAINING AB USING AN ELECTROSPINNING TECHNIQUE. | AMLODIPINE BESYLATE | CURDLAN, POLYETHYLENE OXIDE (PEO), | ELECTROSPINNING
20 KV 1,2 ML/H 18 CM |
THE SOLUBILITY OF CURDLAN WAS IMPROVED AFTER CARBOXYMETHYLATION. AB-LOADED COMPOSITE NANOFIBERS WERE EASILY PREPARED USING A SOLVENT MIXTURE. THE OBTAINED FIBERS WERE SMOOTH AND UNIFORM WITH INCREASING CM-CD CONTENT. THE DRUG WAS DISPERSED IN THE CM-CD/PEO MATRIX IN AMORPHOUS FORM WITH GOOD COMPATIBILITY WITH THE POLYMERS AND INCREASING THE CM-CD CONTENT COULD ALTERED THE RELEASE BEHAVIOR OF THE DRUG. THE FORMULATED DEVICE HAS GOOD CHARACTERISTICS TO BE USED FOR DRUG DELIVERY THROUGH THE BUCCAL MUCOSA. |
| Mann et al.
(2022) |
INDIA | THE MAIN OBJECTIVE OF THE STUDY WAS TO STANDARDIZE AN OPTIMIZED FORMULATION OF POLYVINYL ALCOHOL, SODIUM CARBOXYMETHYL CELLULOSE AND SODIUM ALGINATE THAT WOULD LEAD TO SUSTAINED DRUG DELIVERY IN THE FORM OF BUCCAL PATCHES.
|
VENLAFAXINE HYDROCHLORIDE | POLYVINYL ALCOHOL (PVA)
SODIUM CARBOXYMETHYL CELLULOSE (CMC) SODIUM ALGINATE (SA) |
ELECTROSPINNING
18-20 KV 0,5 ML/H 12 CM |
THE POLYMER CONCENTRATIONS WERE OPTIMIZED IN A MUCOADHESIVE FORMULATION FOR VENLAFAXINE DELIVERY THROUGH THE BUCCAL MUCOSA. THE PATCH HAD OPTIMAL PH FOR ORAL COMPATIBILITY AND PROLONGED RELEASE UP TO 5 HOURS. IN ADDITION, THE POLYMERIC FILM PRODUCED PROTECTED THE DRUG, MAKING IT USEFUL FOR DRUGS SENSITIVE TO ACID DEGRADATION. THE ELECTROPHILIC MEMBRANE REPLICATED SIMILAR BIOCOMPATIBILITY RESULTS WITH A HIGHER DRUG RELEASE RATE. |
|
Alkahtani et al. (2021)
|
LONDON |
THE AIM OF THIS STUDY WAS TO DEVELOP AN ASSOCIATION OF QUETIAPINE (QUE), ESCITALOPRAM (ESC) IN FIXED DOSE AS A RAPIDLY DISSOLVING FILM BY COAXIAL ELECTROSPINNING. |
QUETIAPINE ESCITALOPRAM |
POLYVINYLPYRROLIDONE
|
COAXIAL ELECTROSPINNING 7 – 9 KV 0,5 ML/H 15 CM |
THE NANOFIBERS OBTAINED WITH QUETIAPINE AND ESCITALOPRAM WERE SMOOTH, WITHOUT DEFECTS AND NON-POROUS WITH A DIAMETER OF 0.9 µM, IT WAS ALSO POSSIBLE TO IDENTIFY THE DISTINCT LAYERS OF THE CORE AND THE OUTER PART OF THE FIBER, CONFIRMING THE SUCCESSFUL PREPARATION OF THE FIBERS. BOTH DRUGS WERE DISTRIBUTED IN AN AMORPHOUS WAY AND THE FIBERS HAD A DISINTEGRATION TIME OF 2 S, WHICH ACCELERATED THE RELEASE. THE EX VIVO PERMEABILITY STUDY DEMONSTRATED THAT QUE WAS PERMEATED THROUGH THE BUCCAL MEMBRANE, BUT THE SAME DID NOT HAPPEN WITH ESC PROBABLY DUE TO THE BUCCAL EPITHELIUM AND INTERCELLULAR LIPIDS. HOWEVER, THE USE OF NANOFIBERS FOR THE DELIVERY OF DRUGS IN THE ORAL CAVITY FOR THE TREATMENT OF MENTAL DISORDERS IS PROMISING. |
| Li et al.
(2020) |
CHINA | THE STUDY AIMED TO DEVELOP AND CHARACTERIZE ELECTROSPUN SYSTEMS OF POLYVINYLPYRROLIDONE (PVP), CARVEDILOL AND POLYETHYLENE GLYCOL 400 (PEG 400) FOR SUBLINGUAL DELIVERY OF THE DRUG. | CARVEDILOL (CAR) | POLYVINYLPYRROLIDONE
POLYETHYLENE GLYCOL 400
|
ELECTROSPINNING
10 KV 1,25 ML/H 10 CM |
THIS STUDY DEMONSTRATED THAT ELECTROSPUN FILM OF PVP WITH CAR SHOWED RAPID DISSOLUTION AND IMPROVED PERMEABILITY FOR SUBLINGUAL APPLICATION. THE FLEXIBILITY OF PVP WAS IMPROVED BY MIXING THE POLYMER WITH THE PLASTICIZER PEG, WHICH ALSO FACILITATED THE RELEASE OF CAR. COMPLETE DISSOLUTION WAS DUE TO THE DRUG IN AN AMORPHOUS STATE WHICH WAS CONFIRMED BY THE DSC THERMOGRAM. FTIR SPECTRUM AND MOLECULAR DOCKING SHOWED THAT CAR WAS COMPATIBLE WITH THE EXCIPIENTS DUE TO HYDROGEN BONDING. ELECTROSPUN FILM PROVED TO BE A POTENTIAL ALTERNATIVE FOR SUBLINGUAL RELEASE OF CAR, ESPECIALLY FOR PATIENTS WITH SWALLOWING DIFFICULTIES. |
| Németh et al.
(2020)
|
HUNGRIA | PREPARE CATIONIC POLYASPARTAMIDES MATRICES WITH RAPID DISSOLUTION AT THE PH OF THE ORAL CAVITY VIA ELECTROSPINNING. | VITAMIN B12 | POLYASPARTAMIDES | ELECTROSPINNING:
20 KV 15 CM 0,5 ML/H
|
NANOFIBERS WERE SUCCESSFULLY PREPARED FROM CATIONIC POLYASPARTAMIDES USING THE ELECTROSPINNING TECHNIQUE THAT USED ETHANOL AS A SOLVENT. THE MEMBRANES WERE PRODUCED WITHOUT ADDITIVES, WITH HOMOGENEOUS DISTRIBUTION AND NANOMETRIC SIZE. THE POLYMER CONCENTRATION IN THE MEMBRANE WAS 25% AND THE MEMBRANE DISSOLVED ALMOST COMPLETELY IN ONE MINUTE IN ARTIFICIAL SALIVARY FLUID (PH = 6.8), AND INCORPORATED VITAMIN B12 WAS RAPIDLY RELEASED. THESE RESULTS SUGGEST THE APPLICABILITY OF ELECTROSPUN FILMS OF POLYASPARTAMIDES IN SUBLINGUAL AND ORAL ADMINISTRATION OF DRUGS. |
| Qin et al.
(2019) |
CHINA | PRODUCE FAST-DISSOLVING ORAL NANOMETRIC FILMS VIA ELECTROSPINNING. | ASPIRIN | PULLULAN
POWDERED CHITOSAN |
ELECTROSPINNING
15 KV 0,5 ML/H 10 CM |
NANOFIBER MEMBRANES WITH DIFFERENT PROPORTIONS OF PULLULAN AND CHITOSAN WERE SUCCESSFULLY MANUFACTURED AND THE SEM DEMONSTRATED THAT THE FIBER DIAMETER DECREASED WITH THE INCREASE OF CHITOSAN IN THE SOLUTIONS AND THE INTERACTION AND MISCIBILITY OF THE POLYMER CHAINS WAS PROVEN AND A RAPID DISSOLUTION TEST WAS PERFORMED WITH THE ENCAPSULATION OF THE DRUG ACETYL SALICYLIC ACID TO DEMONSTRATE ITS FAST DISSOLUTION CAPACITY AND GREAT POTENTIAL TO BE USED IN THE ORAL MUCOSA. |
| Chen et al.
(2019) |
CHINA | DEVELOP TWO TYPES OF COAXIAL FIBERS WITH CMCS, CMC AND LIPOSOMES TO IMPROVE THE BIOAVAILABILITY OF CARVEDILOL. | CARVEDILOL | CARBOXYMETHYLCHITOSAN
POLYVINYL ALCOHOL (PVA) POLYVINYL PYRROLIDONE (PVP) SODIUM CARBOXYMETHYL CELLULOSE (CMC-NA) |
ELECTROSPINNING
12,5 KV 0,4 – 0,13 ML/H 10 CM |
IN THIS STUDY, THROUGH COAXIAL ELECTROSPINNING, UNIFORM FIBERS WERE OBTAINED, WITH A DIAMETER OF APPROXIMATELY 200 NM. THE BIOADHESIVE POLYMER IMPROVED IN VITRO ADHESIVE STRENGTH OF THE FIBERS AND IN PERMEATION TESTS USING THE TR146 CELL MODEL AND PORCINE ORAL MUCOSA, THE DATA SHOWED THAT LIPOSOMES HAD A POSITIVE EFFECT ON PERMEATION. IN SUMMARY, THE IDEA OF COMBINING LIPOSOMES AND NANOFIBERS FROM BIOADHESIVE POLYMERS WAS FAVORABLE TO THE ORAL ABSORPTION OF CARVEDILOL. |
| Chen et al.
(2018a) |
CHINA | DEVELOP MULTILAYER LIPOSOMAL SYSTEM VIA ELECTROSPINNING PROCESS TO IMPROVE THE BIOAVAILABILITY OF CARVEDILOL. | CARVEDILOL | POLYVINYL PYRROLIDONE
HYDROXYPROPYLMETHYL CELLULOSE SODIUM CARBOXY METHYL CELLULOSE |
ELECTROSPINNING
9 KV 1,5 ML/H 15 CM |
THE ASSOCIATION OF NANOPARTICLES AND ELECTROSPINNING ALLOWED, IN AN IN VITRO ENVIRONMENT, THE SPONTANEOUS FORMATION OF THE LIPOSOME/CARVEDILOL IN SITU WHEN THE SPUN FIBER CAME INTO CONTACT WITH WATER. THIS PROJECT CIRCUMVENTED THE DISADVANTAGE OF THE LIPOSOME SOLUTION AND INCREASED ITS SHELF LIFE. FURTHERMORE, THE MUCOADHESIVE LAYER WAS USED TO FURTHER PROLONG THE RETENTION TIME OF THE PREPARATION ON THE MUCOSAL SURFACE, THUS IMPROVING ITS BIOAVAILABILITY. THE STUDY CONFIRMED LIPOSOME FORMATION AND EXCELLENT PERMEABILITY THROUGH THE PORCINE ORAL MUCOSA. IN VIVO, THE PHARMACOKINETIC STUDY OF THE MUCOADHESIVE FIBROUS MEMBRANE IN THE RABBIT ALSO SHOWED EXCELLENT IMPROVEMENT IN BIOAVAILABILITY COMPARED TO CAR SUSPENSION. BASED ON THE EXPERIMENTAL RESULTS, IT WAS CONCLUDED THAT THE MUCOADHESIVE FIBROUS FILM WAS ABLE TO ADMINISTER DRUGS WITH LOW ORAL BIOAVAILABILITY. |
| Kazsoki et al.
(2018) |
HUNGRIA | THE AIM OF THIS WORK WAS TO SHOW THE EFFECTS OF DIFFERENT FORMULATIONS (FILM AND FIBER FORMATION) ON THE MORPHOLOGY OF PAPAVERINE IN GEL MATRIX. | PAPAVERINE-HCL | HYDROXYPROPYL CELLULOSE
POLY(VINYL ALCOHOL)
|
ELECTROSPINNING
30 KV 15 CM |
THE RESULTS OF THIS STUDY HIGHLIGHT THE IMPORTANCE OF SUPRAMOLECULAR CHANGES DURING THE GEL-FILM TRANSITION INDUCED BY DRYING OR GEL-FIBER FORMATION. THE ANOMALOUS GEL-FILM TRANSITION MAY BE RELATED TO THE PRESENCE OF INTRAMOLECULAR WATER THAT COULD INITIATE THE AMORPHOUS-CRYSTALLINE TRANSITION OF THE DRUG. THIS SUGGESTS THAT THE MAPPING OF POLYMER-DRUG AND POLYMER-WATER BINDING ENERGIES IS ESSENTIAL FOR SELECTING A POLYMER TO ACT AS A DRUG RELEASE MATRIX. |
| Nazari et al.
(2017) |
GRECIA/
CHINA |
DEVELOP INDOMETHACIN FILMS FROM A POLYMER BLEND USING THE ELECTROSPINNING TECHNIQUE FOR DRUG DELIVERY IN THE BUCCAL MUCOSA. | INDOMETHACIN | POLYVINYLPYRROLIDONE
METHOCEL E5 METHOCEL E15
|
ELECTROSPINNING
15 KV 20µL/MIN 15 CM
|
THE RESULTS SHOWED THAT VARIOUS FORMULATIONS OF ELECTROSPUN MOUTHWASHES CONTAINING INDOMETHACIN CAN BE EASILY OBTAINED. THE ADDITION OF TWEEN® 80 TO PVP-INDO FORMULATIONS SIGNIFICANTLY IMPROVED FIBER WETTABILITY. ADHESIVES CONTAINING METHOCEL AND TWEEN® 80 EXHIBITED THE GREATEST PERMEATION OF INDOMETHACIN THROUGH THE PORCINE MUCOSA, WITHOUT SIGNIFICANTLY AFFECTING THE STRUCTURE OF THE ORAL MUCOSA. |
| Kazsoki; Szabó; Zelkó.
(2017) |
HUNGRIA | PRODUCE FIBERS VIA THE ELECTROSPINNING TECHNIQUE WITH PAPAVERINE FOR ORAL ADMINISTRATION, AIMING TO IMPROVE THE BIOAVAILABILITY OF THE CRYSTALLINE DRUG. | PAPAVERINE-HCL | HYDROXYPROPYL CELLULOSE
POLY(VINYL ALCOHOL) |
ELECTROSPINNING
20, 25 E 30 KV 0,2 E 0,3 ML/H 5, 10 E 15 CM |
HPC, PVA AND PAPAVERINE HYDROCHLORIDE NANOFIBROUS SYSTEMS WERE PREPARED SUCCESSFULLY BY ELECTROSPINNING PROCESS. THIS FORMULATION WAS ABLE TO ALLOW THE RELEASE OF AMORPHOUS DRUG THROUGH THE ORAL MUCOSA, IMPROVING THE BIOAVAILABILITY OF PAPAVERINE WITHOUT INTERFERING WITH FIRST-PASS METABOLISM. |
| Frizzell; Ohlsen; Woosrow
(2017) |
EUA | DEVELOP AN EMULSION ELECTROSPINNING METHOD FOR PROTEIN TRANSPORT IN EUDRAGIT FIBERS. | HORSERADISH PEROXIDASE (HRP), ALKALINE PHOSPHATASE | EUDRAGIT® L 100
POLY(VINYL ALCOHOL) |
ELECTROSPINNING
12,5 KV 25 E 40 µL MIN -1 10-12 CM |
HORSERADISH PEROXIDASE AND ALKALINE PHOSPHATASE PROTEINS WERE INCORPORATED INTO EUDRAGIT® L100 EMULSION FIBERS BY ELECTROSPINNING PROCESS. IT WAS OBSERVED THAT THE FLOW RATE DURING ELECTROSPINNING HAS NO EFFECT ON PROTEIN BIOACTIVITY, DEMONSTRATING THAT THE ELECTROSPINNING PROCESS IS NOT HARMFUL TO THE PROTEIN AND INCREASES THE STABILITY OF THE FORMULATION AFTER LYOPHILIZATION, INDICATING THAT IT IS A NEW AND PROMISING PLATFORM FOR RELEASE OF PROTEINS THROUGH THE ORAL ROUTE WITHOUT USING THE GASTROINTESTINAL TRACT. |
| Masek et al.
(2017) |
CZECH REPUBLIC | THE AIM OF THIS STUDY WAS TO DEVELOP PLGA/PEG ELECTROSPUN FILM FOR USE IN VACCINATION VIA THE SUBLINGUAL ROUTE. | PLGA
PEG HYDROXYPROPYL METHYLCELULOSE CHITOSAN POLYCAPROLACTONE
|
ELECTROSPINNING
50 KV 10 CM |
THIS STUDY DEMONSTRATED IN AN EX VIVO AND IN VIVO ANIMAL MODEL HOW ELECTROSPUN NANOFIBROUS MUCOADHESIVE FILMS CAN SERVE AS PROTECTIVE RESERVOIRS FOR CONTROLLED AND SUSTAINED RELEASE NANOPARTICLES IN THE SUBMUCOSAL TISSUE. FURTHERMORE, IT HAS BEEN SHOWN THAT FILMS CAN BE PREPARED IN DIFFERENT WAYS FROM VARIOUS POLYMERIC MATERIALS FOR USE WITH MANY DIFFERENT TYPES OF NANOPARTICLES SUCH AS LIPOSOMES, VIRUSES AND SIMILAR PARTICLES, BIOPOLYMERS, MOLECULAR ADJUVANTS AND PHARMACEUTICAL EXCIPIENTS. | |
| Hosseinzadeh et al.
(2016) |
IRAN | THIS STUDY AIMED TO DEVELOP A COMPOSITE NON-WOVEN MESH CONTAINING ZIZIPHUS JUJUBA EXTRACT THROUGH ELECTROSPINNING AND THE RELEASE PROFILE WAS STUDIED IN ARTIFICIAL SALIVA. | ZIZIPHUS JUJUBA | CHITOSAN
POLYETHYLENE OXIDE (PEO), |
ELECTROSPINNING
18 KV 1 ML/H 14 CM |
NANOFIBERS HAVE A GREAT POTENTIAL TO ACCELERATE RELEASE KINETICS WHICH IS CONSIDERED AN IMPORTANT FACTOR IN PERIODONTAL DISEASES. HERE, THE PROPOSED CHITOSAN-PEO/PHENOLIC COMPOUND NANOFIBER NETWORK PROVIDED THE ACTIVE RELEASE OVER 75 MIN.
IN THIS SENSE, THERE WAS AN ADEQUATE SWELLING OF THE SYSTEM, WHICH FACILITATED THE DESIRED RELEASE OF THE DRUG. |
| Borbás et al.
(2015)
|
HUNGRIA | DEVELOP A SULFOBUTHYLETHER- B-CYCLODEXTRIN-BASED ELECTROSPUN FORMULATION TO INVESTIGATE THE IN VITRO DISSOLUTION AND PERMEATION PROPERTIES OF A COMPLEX FORMULATION MATRIX USING MFLUX | ARIPIPRAZOLE | SULFOBUTHYLETHER-
B-CYCLODEXTRIN POLY(ETHYLENE OXIDE) |
ELECTROSPINNING
40 KV 2ML/H 50 CM |
THE FORMULATION BASED ON ELECTROSPUN CYCLODEXTRIN WITH ARIPIPRAZOLE SHOWED THE POTENTIAL TO RELEASE THE DRUG THROUGH THE ORAL MUCOSA DUE TO THE ULTRA-RAPID DISSOLUTION POSSIBLE THROUGH THE NANOFIBERS EVEN IN A SMALL VOLUME OF THE DISSOLUTION MEDIUM. THESE RESULTS PREDICT SIGNIFICANTLY INCREASED BIOAVAILABILITY FOR THE FORMULATION VIA ELECTROSPINNING. THE DIFFERENCE IN THE RESULTS OF THE DISSOLUTION AND DISSOLUTION-PERMEATION EXPERIMENTS DEMONSTRATES THAT WITH THE DISSOLUTION-PERMEATION TESTS CARRIED OUT WITH THE MFLUX DEVICE IT IS POSSIBLE TO PREDICT THE BIOAVAILABILITY MORE ACCURATELY, BECAUSE WITH THESE RESULTS NOT ONLY DISSOLUTION, BUT THE INTERACTION BETWEEN SOLUBILITY AND PERMEABILITY IS ALSO CONSIDERED. |
| Aduba Junior. et al. (2013) | USA | FABRICATE GELATIN NANOFIBER SCAFFOLDS VIA ELECTROSPINNING AND EXPLORE THEM FOR LOCAL DELIVERY OF THERAPEUTICS FOR CANDIDIASIS. | NYSTATIN | GELATINA
POLIETILENOGLICOL DIACRILATO |
ELECTROSPINNING:
25 KV 7,5 CM 5 ML/H |
THIS STUDY DEVELOPED GELATIN SCAFFOLDS CROSSLINKED WITH PEG-DA575 AND OBTAINED NANOFIBERS WITH SATISFACTORY MORPHOLOGY. IT WAS FOUND THAT THE STRUCTURAL PARAMETERS OF THE NANOFIBERS CAN BE MODULATED BY CHANGING THE CONCENTRATION OF THE CROSSLINKING AGENT AND THIS CONTRIBUTED TO INCREASE THE STABILITY OF THE NANOFIBERS. |
Source: The author, 2023.
3.1 NANOFIBER SCAFFOLDS FOR BUCCAL DRUG DELIVERY
Electrospun fibers have been widely studied as scaffolds in the most varied biomedical areas. In the development of delivery systems through the buccal mucosa, nanostructures can increase the drug’s solubility, avoid first-pass metabolism, increase bioavailability and promote the continuous release of the active (Shirvan; Bashari; Hemmatinejad, 2019).
In the study by Aduba Junior. et al. (2013) gelatin scaffolds were produced using the electrospinning for the topical release of nystatin in the buccal cavity, aiming at the treatment of candidiasis. The authors aimed to develop structures with high surface area, good adhesion directly at the site of infection, ease in adjusting the physical properties of the polymer used, to control drug release and maintain its structural integrity. However, nanofibers produced from gelatin have low stability in aqueous phases, which becomes a problem when in contact with saliva in the oral cavity. To solve this problem, the authors used an agent as an alternative crosslinker not yet studied in the crosslinking of nanofiber scaffolds, polyethylene glycol diacrylate (PEG-DA) (Sell et al., 2008).
Treatment with PEG-DA is able to improve both the water resistance capacity and minimally affects the morphology of nanofibers. However, the results obtained showed that although the diameter of the nanofibers was affected by the crosslinking agent and by the addition of ethanol as a means to facilitate crosslinking, no significant morphological difference was observed.
The mucoadhesive property was also confirmed through a scaffold mucin absorption test and the release of the nystatin occurred in its entirety after 120 hours. Considering that an ideal system for drug administration in the oral mucosa should release the drug within 4 to 6 hours, due to the mucin turn over, food and liquid intake and saliva production, further studies are needed to optimize the time of drug release in the buccal cavity (Aduba Junior. et al., 2013).
Nazari et al. (2017) developed nanometric films by the electrospinning process, using Polyvinylpyrrolidone (PVP), Methocel™ (E5) premium, Methocel™ (E15) and Tween® 80 as polymers, and incorporated the drug indomethacin. The films exhibited a smooth face, but with variable diameter. This variation could be attributed to the inclusion of excipients and the proportion of co-polymers. The X-ray diffraction analysis found that the indomethacin was initially in crystalline form and after electrospinning, was dispersed in the fibrous matrices, in an amorphous state, which favors rapid release and increased solubility.
TGA and DSC were used to characterize the obtained films and it was verified the passage of indomethacin from the crystalline to the amorphous state after electrospinning. The measurement of the contact angle allowed note that the inclusion of Tween® 80 increases the hydrophilic property of the membranes. The Indomethacin release behavior of membranes was observed and the authors verified that the presence of methylcellulose results in a decrease in the release rate, due to its low solubility in the medium (Shi; Lu; Jiang, 2009). In this study, the researchers concluded that the addition of Tween® 80 to the PVP-INDO formulations significantly improved membrane swelling and those containing Methocel and Tween® 80 exhibited the greatest permeation of INDO across the porcine mucosa.
In the study of Kazsoki et al. (2018) the authors evaluated the effects of different formulations on the morphology of papaverine in gel matrix and found that NMR spectroscopy identified the nanocrystals that formed in the drying process of the films. In contrast, the same nanocrystals were below the detection limits of infrared spectroscopy, demonstrating the change in morphology in the structure of papaverine.
The results found highlighted the importance of the molecular changes that occurred in the gel-film transition process and showed that the anomalous transition may be related to the presence of secondary intramolecular water binding, which can initiate the amorphous-crystalline transition of the drug. This suggests that the mapping of polymer-drug and polymer-water binding energies is essential for the development of a drug delivery system.
In the study of Hosseinzadeh et al. (2016) a non-woven mesh containing Ziziphus jujuba extract was prepared and the release profile was studied in artificial saliva. The polymers used were chitosan and polyethylene oxide aiming at greater hydrophilicity and greater mucoadhesiveness.
The phenolic compound extracted from Z. jujuba was chosen for the study because of its anti-inflammatory and antibacterial properties. The morphology and chemical state of the film were investigated using Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR), respectively. The film obtained from chitosan/PEO promoted the release of the impregnated phenolic compound in 75 minutes. The Fickian diffusion of phenolic compound release demonstrated the desirable release of the active. The authors also found that the swelling capacity of the polymer played an important role in the release rate of the drug, as it created a hydrophilic and erosional condition, thus facilitating the release.
Stie et al. (2023) investigated a multi-layer system (nanofiber/foam/film) using a combination of polysaccharides. The system obtained showed excellent mechanical properties and biocompatibility. The chitosan-based nanofibers provided the system with better mucoadhesion and the authors found that, after 1 h, more than 80% of the active ingredient had been released from the formulation. Ex vivo permeation studies using the oral mucosa of pigs revealed that the formulation developed increases the permeation of desmopressin compared to the pharmaceutical form currently marketed. The results showed that the system is promising for use as a pharmaceutical form.
The study by Mann et al. (2022) formulated a system composed of polyvinyl alcohol, sodium carboxymethylcellulose and sodium alginate with the aim of producing a sustained release system for the drug. The adhesives showed high tensile strength with uniform drug content. The drug release was stable, reaching a maximum release of 89.97% in 5 h. The absence of hemolysis and toxicity in TR146 cells at various concentrations demonstrated the mucoadhesive’s biocompatibility with human tissues. The in vitro and in vivo data showed that the formulation developed allowed sustained release of the drug for about 5 continuous hours, highlighting the potential of the polymeric combination in research into sustained drug delivery systems.
3.2 FAST DISSOLVING FILMS PREPARED FROM ELECTROSPINNING
Fast-dissolving oral films are flexible, non-friable polymeric films that contain one or more dispersed drugs. This type of pharmaceutical form is intended for application in the buccal region aiming at rapid disintegration or dissolution in saliva (Sultana; Arafat; Pathan, 2013; Slavkova; Breitkreutz, 2015). This type of system is quickly dissolved in saliva, without the need for swallowing or chewing, which facilitates use by geriatric, pediatric or bedridden patients (Goldberg; Gomez-Orellana, 2003; Qin et al., 2019).
In the study of Qin et al. (2019) the authors proposed the development of fast-dissolving oral films of natural polysaccharides (chitosan and pullulan) and acetylsalicylic acid as a drug. When evaluating viscosity, the authors found that all prepared solutions showed pseudoplastic behavior and the average fiber diameter decreased with the increase in chitosan content up to a ratio of 30/70 (chitosan/pululan), however, the diameter of the nanofibers began to increase as the amount of chitosan in the solution increased.
The FTIR spectra of different samples showed that hydrogen bonds were formed between polymer chains during the electrospinning. However, the authors also found that the hydrogen bonds between the OH groups of pullulan and the NH groups of chitosan affected the displacement of the stretch peaks.
When evaluating X-ray diffraction, the authors demonstrated that both chitosan and pullulan did not present their own diffraction, which demonstrated interaction and miscibility between the polymers. The calorimetric analysis showed that the C/P 0/100 nanofiber film was more thermally stable than the pullulan powder, indicating that the intermolecular interaction was increased. Furthermore, with the increase in chitosan content, the C/P nanofiber films showed greater thermal stability due to the hydrogen bond between the OH groups of pullulan and NH2 of chitosan. Another analysis performed in the study was the dissolution of the mats in water, and complete dissolution was found in 60 seconds due to the properties of the two polysaccharides, the high surface area and porosity of the fibers.
Acetylsalicylic acid is poorly soluble in water and absorbed mainly from the intestinal tract, but some particles may remain attached to the gastric mucosa, causing erosion, ulceration, bleeding and even gastric perforation. Therefore, encapsulating aspirin in fast-dissolving films to release the drug into the buccal cavity can prevent adverse effects (Farias; Boateng, 2018). To demonstrate the presence of acetylsalicylic acid in the fibers, samples were subjected to UV-visible absorption, and films without the drug showed no absorption. The authors concluded that the tests evaluated proved the ability of C/P nanofiber films to encapsulate drugs for various applications.
Alkahtani et al. (2021) evaluated a fixed-dose combined formulation of Escitalopram (ESC) and Quetiapine (QUE) in order to obtain a film of rapid dissolution and consequent release of drugs through the oral mucosa. The films produced presented fibers with uniform morphology and distribution, smooth, cylindrical and non-porous. DSC and XRD analyzes performed showed that the drug loaded fibers were in the form of solid amorphous dispersion. In the FTIR analysis, compatibility between the fiber components was found and the interaction between them tended to increase the long-term stability. The disintegration time in the simulated salivary fluid was 2 seconds according to studies using similar polymers. Analysis of the drug’s release showed that more than 50% of ESC and QUE were released after 5 minutes and that total release was observed after 120 minutes. However, the ex vivo study on the oral bovine membrane’s found only the permeation of QUE. This may be due to the structure of the oral epithelium tested and further studies need to be carried out to increase ESC permeability.
3.3 SUBLINGUAL ADMINISTRATION ROUTE AND THE NANOFIBERS AS A DRUG CARRIER
The sublingual route of drug administration refers to the administration of medication under the tongue. The sublingual route presents rapid absorption, but the drug’s duration of action is short. In terms of permeability, the sublingual route has high permeability and formulations of various drugs have sublingual bioavailability comparable to oral bioavailability (Sandri et al., 2020).
Németh et al. (2020) developed cationic polyaspartamide matrices and found that the type of cationic group had a significant effect on fiber formation and moisture absorption, and by increasing polymer chain length, fiber formation improved. The authors also found that fiber size and shape vary considerably with polymer concentration. Electrospinning of low-concentrated solutions generally produces fibers that stick together along the way, forming ribbons due to incomplete solvent evaporation before the polymer reaches the collector (Zubir, 2020). The study showed that the ideal concentration of polymers for obtaining nanofibers was 25 wt% and FTIR found that the polymer does not undergo chemical degradation during electrospinning and is stable. The X-ray demonstrate that the vitamin B12 (model drug) and the polymeric matrices were in the amorphous state. Complete release of vitamin B12 was observed within one minute due to the rapid dissolution of the matrices in simulated salivary fluid, which demonstrates a possible application of the matrix as a sublingual release system.
Mašek et al. (2017) by studying that the oral mucosa it was found that non-keratinized oral regions are appropriate sites for the administration of drugs and immunizing agents. In this sense, they proposed the combination of layers of electrospun fibers with mucoadhesive layers for the release of drugs in the sublingual and oral mucosa.
The authors developed a mucoadhesive film with three distinct layers: the nanofiber reservoir layer, where nanoparticles were deposited on the surface of the nanofibers or in the pores between these, the mucoadhesive layer to keep the system fixed at the application site and the support layer that was intended to prevent the diffusion of nanoparticles out of the application site and protect the system from the shear effects of the buccal cavity and the saliva flow. The ex vivo and in vivo study showed that the films produced played the role of protection and reservoir of nanoparticles and it was possible to promote the release of the active in a controlled way in the mucosa.
Li et al. (2020) prepared Polyvinylpyrrolidone (PVP) nanofibers loaded with carvedilol (PVP) and Polyethylene Glycol 400 (PEG 400) for sublingual release. The results presented by the authors showed that carvedilol appeared in the amorphous form after the electrospinning process and the fibers were cylindrical and smooth with a mean diameter of 745 ± 57 nm. The films showed fast dissolution and good permeability to be used through the sublingual route. The flexibility of PVP was improved with the addition of plasticizer agent (PEG), which also facilitated the release of carvedilol.
3.4 USE OF EXCIPIENTS TO ENHANCE THE DISSOLUTION AND THE BIOAVAILABILTY OF A DRUG AND ELECTROSPINNING PROCESS
Electrospinning when combined with other pharmaceutical technologies such as surfactants, liposomes, nanoparticles and cyclodextrins can significantly improve the results of increased permeation and absorption of drugs in the buccal cavity (Muppalaneni; Mastropietro; Omidian, 2013; Sagitha, et al.2021).
In the study by Chen et al. (2018a), the researchers produced films, where PVP acted as a filament-forming matrix and phospholipid molecules were added for the in-situ formation of liposomes. They proposed the development of a triple mucoadhesive film with the following layers: support, mucoadhesive and nanofiber layer. The drug chosen for the study was carvedilol, that it has low oral bioavailability, around 25%-35%. The authors found that in an in vitro, the carvedilol liposome formed spontaneously when the fibers came into contact with water. The use of the mucoadhesive layer made it possible to further prolong the retention time of the film on the oromucous surface, improving bioavailability and in vivo tests in rabbits also showed improvement in bioavailability, suggesting this to be a promising system for releasing drugs with low solubility.
Chen et al. (2019) in the following year, proposed the development of a multilayer electrospun film, however, using the coaxial electrospinning technique to increase the solubility of the drug carvedilol. According to the study design, the film when placed in the mouth, the outer layer should be able to adhere to the mucosa, and the inner drug layer should form the liposome in situ after water absorption and promote mucosal permeation. Fibers were electrospinned and obtained uniform fibers, with a diameter of approximately 200 nm. XRD revealed that carvedilol was in an amorphous state, the fibers had a linear dissolution profile in the first 2 hours without drug release, which reveals potential for slow-release formulation. The bioadhesive polymer improved the adhesive strength in vitro and in permeation tests, the data showed that the liposome formed in situ increased the drug permeation.
Another excipient widely used in the pharmaceutical industry are cyclodextrins because these cyclic oligosaccharides can act as solubilizing and stabilizing agents due to the formation of inclusion complexes with lipophilic substances (Yarin, 2011). In the study of Borbás et al. (2015) the researchers investigated in vitro permeation and dissolution properties of formulation using µfluxTM apparatus. For the formation of fibers, the polymer used was ethylene oxide and the authors obtained fine fibers without defects. The drug analyzed in the fibers was found in an amorphous state and no crystallization was observed even after 3 months and the study showed that the formulation based on electrospun cyclodextrin had the potential to ensure the rapid release of the drug through the oral mucosa even in a small volume of dissolution medium as shown by the results of the in vitro dissolution and permeation test.
In the study by Zhao et al. (2022) were prepared composite nanofibers using carboxymethylated curdlan (CM- CD) to improve the solubility of curdlan. They also used Amlodipine Besylate (AB) formulated with Poly(Ethylene Oxide) (PEO) and CM-CD in orodisperse Nanofiber Films (NOFs) by electrospinning. NMR and FTIR showed that modified CM-CDs were obtained and that it was possible to produce solutions that electrofied uniformly using a water/methanol solvent mixture. The diameter of the composite nanofibers was about 200-300 nm and with a smooth surface. The drug was amorphous and distributed throughout the electrophilic membrane without interacting with the polymer. Based on the results obtained, the authors propose that CM-CD-based nanofibers are promising platforms for drug delivery into the oral mucosa.
In the study by Friedl and colleagues (Friedl et al., 2022), electrospun systems were evaluated with the aim of combining the benefits of mucoadhesive fibers and systems known as “Self-Emulsifying Drug Delivery Systems (SEDDS)”. The adhesives produced allowed the encapsulation of active ingredients, reduced the diameter of the fibers and provided controlled release of curcumin for more than 3 hours. The addition of thiolated polyacrylic increased the buccal residence time of the adhesives by 200-fold and significantly increased the penetration of the drug into the buccal tissue compared to fiber adhesives without SEDDS. The adhesives were classified as biocompatible and showed the therapeutic effects on human keratinocytes and fibroblasts. The produced electrophilic mucoadhesive represent an alternative for lipophilic drug delivery.
3.5 EMULSION ELECTROSPINNING
Proteins are extremely sensitive macromolecules, since they undergo changes in their conformation in the presence of ultraviolet light, pH variations, temperature rises and, when in solution, they interact with organic solvents. In this sense, so that it is possible to carry out electrospinning of proteins without interfering with their biological activity, an alternative is to carry out electrospinning by emulsion (Goldberg; Gomez-Orellana, 2003; Gupta et al., 2013).
Frizzell, Ohlsen e Woodrow (2017) in their study developed electrotrospun fibers from a protein emulsion aiming to maintain biological activity and controlled release. The nanofibers were produced as proposed by Yarin (2011), from an emulsion composed of an organic phase (containing polymer), a surfactant and an aqueous phase (containing protein). The fibers formed had a continuous core surrounded by a viscous polymer shell, a structure that increases protein stability and controlled release when compared to solution electrospinning that directly exposes proteins to organic solvents and exhibits release kinetics fast (Ji et al., 2011). In this study, horseradish peroxidase and alkaline phosphatase were incorporated into Eudragit L100® fibers during the emulsion electrospinning process. The authors observed that the proteins were incorporated into the fibers and the release analysis showed controlled release at different pHs. In addition, freeze-drying the fibres obtained increased the product’s shelf life at room temperature by 7 days.
4. CHALLENGES AND PERSPECTIVES IN THE DEVELOPMENT OF DRUG DELIVERY SYSTEMS FOR THE ORAL CAVITY
In recent years it has been possible to notice enormous progress in the area of electrospinning. Currently is recognized as a technique capable of processing a series of materials into nanofibers with controlled diameters. The morphology and internal structure of these fibers can also be measured using physical and chemical methods, and in addition, variations in the method, such as coaxial electrospinning, emulsion electrospinning, fiber alignment, among others, are employed for different purposes (Han et al., 2022; Mann et al., 2022).
The evolution of research in the area has allowed the use of electrospinning for the production of nanofibers in several areas, especially in the biomedical area, but some challenges need to be faced, such as: control of the size and morphology of electrospun fibers, since electrospinning fibers with smaller sizes that 100 nm is no easy task. In addition, materials subject to electrospinning require further studies, because although a large number of polymers, mainly, are being used, the addition of another polymer to the solution, forming blends, can often make it possible to obtain more functional fibers (Mohammadinejad et al., 2021; Li; Xia, 2004).
Another factor to be considered is the low production rate. Currently, most studies are carried out on equipment that does not have industrial-scale production capacity, which generates a low production rate, and high cost per gram of nanofibers and the use of organic solvents that bring economic and environmental concerns (Aytac; Uyar, 2022).
Systems for drug delivery in the buccal cavity have several advantages when compared to conventional systems, such as ease of administration, accessibility, favorable bioavailability and reduction of some side effects. However, the development of these systems requires dedication so that it is possible to obtain pharmaceutical formulations with specific characteristics such as: biocompatibility, mucoadhesiveness, capacity for sustained drug release to maintain favorable plasma levels, in addition to patient acceptance of use (Irfan, et al., 2016; Behere; Ingavle, 2022).
Studies that evaluate cell viability, in vitro and in vivo bioadhesiveness, drug release assays, material design and especially clinical application need to be considered, since they are factors that determine the effectiveness of the treatment (Rasouli et al., 2019).
5. CONCLUSION
Electrospinning has been recognized in the scientific community as a simple and versatile method for the production of nanofibers from synthetic, natural and hybrid polymers. The technique has functional properties and a significant increase in the surface area of the film, which enables better fixation of the material to the cells, incorporation of drugs and makes the obtained system promising for the development of pharmaceutical forms. Drugs ranging from vitamins, antihypertensives, to proteins, can be incorporated into the electrotrospun fibers and their release occurs by simple diffusion or by scaffold degradation. The development of new electrospinning techniques, such as electrospinning with multiple syringes, coaxial electrospinning and emulsion electrospinning has allowed further improvement of the fibers obtained and the control of porosity, morphology, diameters and drug release kinetics, in addition to masking the bitter taste of drugs due to the multilayer structure, making the system efficient for oral use.
The infinite possibilities of electrospinning allow the development of innovative drug delivery systems capable of maximizing the therapeutic benefits of drugs while minimizing their unwanted side effects. The choice of drug and polymers used must be carried out according to the place of application and specific characteristics and each component. All the articles presented in this study demonstrated the important role of the electrospinning process in the development of drug delivery systems in the buccal cavity and the varied and mainly adjustable characteristics of this method allow a wide variety of use, which makes it simple, useful and inexpensive for the incorporation of therapeutic agents.
REFERENCES
ADUBA JUNIOR., Donald C. et al. Semi-interpenetrating network (sIPN) gelatin nanofiber scaffolds for oral mucosal drug delivery. Acta Biomaterialia, v. 9, n. 5, p. 6576–6584, 2013. DOI: https://doi.org/10.1016/j.actbio.2013.02.006.
ALAZAB, Mohamed et al. Sustainable Electrospinning of Nanoscale Fibres. Procedia Manufacturing, v. 12, p. 66-78, 2017. DOI: https://doi.org/10.1016/j.promfg.2017.08.009.
AL-HAZEEM, Nabeel Zabar Abed. Nanofibers and Electrospinning Method. In: KYZAS, George Z. (ed.). Novel Nanomaterials: Synthesis and Applications. London: IntechOpen, 2018. chapter. 11.
ALKAHTANI, Manal E. et al. Fabrication and characterization of fast-dissolving films containing escitalopram/quetiapine for the treatment of major depressive disorder. Pharmaceutics, v. 13, n. 6, p. 891, 2021. DOI: https://doi.org/10.3390/pharmaceutics13060891.
ANSARI, Mudassir; SADARANI, Bhakti; MAJUMDAR, Anuradha. Optimization and evaluation of mucoadhesive buccal films loaded with resveratrol. Journal of Drug Delivery Science and Technology, v. 44, p. 278–288, 2018. DOI: https://doi.org/10.1016/j.jddst.2017.12.007.
AYTAC, Zeynep; UYAR, Tamer. Electrospun Nanofibers for Drug Delivery Applications. In: ANDRADY, Anthony L.; KHAN, Saad A. (eds). Applications of Polymer Nanofibers. Wiley, 2022. Chapter 6. DOI: https://doi.org/10.1002/9781119267713.ch6.
BEHERE, Isha; INGAVLE, Ganesh. In vitro in and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J Biomed Mater Res Part A, v. 110, n. 2, p. 443–461, 2022. DOI: https://doi.org/10.1002/jbm.a.37290.
BHARDWAJ, Nandana; KUNDU, Subhas C. Electrospinning: A fascinating fiber fabrication technique. Biotechnology Advances, v. 28, n 3, p. 325-347, 2010. DOI: https://doi.org/10.1016/j.biotechadv.2010.01.004.
BORBÁS, Eniko et al. In vitro dissolution-permeation evaluation of an electrospun cyclodextrin-based formulation of aripiprazole using μFluxTM. International Journal of Pharmaceutics, v. 491, n. 1-2, p. 180–189, 2015. DOI: https://doi.org/10.1016/j.ijpharm.2015.06.019.
CARVALHO, Flavia Chiva; CHORILLI, Marlus; GREMIÃO, Maria Palmira D. Plataformas bio(muco) adesivas poliméricas baseadas em nanotecnologia para liberação controlada de fármacos – propriedades, metodologias e aplicações. Polimeros, v. 24, n. 2, p. 203–213, 2014. DOI: http://dx.doi.org/10.4322/polimeros.2014.043.
CHEN, Jianting et al. Self-assembled liposome from multi-layered fibrous mucoadhesive membrane for buccal delivery of drugs having high first-pass metabolism. International Journal of Pharmaceutics, v. 547, n. 1-2, p. 303–314, 2018a. DOI: https://doi.org/10.1016/j.ijpharm.2018.05.062.
CHEN, Jianting et al. Two types of core/shell fibers based on carboxymethyl chitosan and Sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. International Journal of Biological Macromolecules, v. 128, p. 700–709, 2019. DOI: https://doi.org/10.1016/j.ijbiomac.2019.01.143.
CHEN, Shixuan et al. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Advanced Drug Delivery Reviews, v. 132, p. 188-213, 2018b. DOI: https://doi.org/10.1016/j.addr.2018.05.001.
CONTRERAS-CÁCERES, Rafael et al. Electrospun nanofibers: Recent applications in drug delivery and cancer therapy. Nanomaterials, v. 9, n. 4, p. 1-24, 2019. DOI: https://doi.org.10.3390/nano9040656.
DEEPAK, Amar; GOYAL, Amit Kumar; RATH, Goutam. Nanofiber in transmucosal drug delivery. Journal of Drug Delivery Science and Technology, v. 43, p. 379-387, 2018. DOI: https://doi.org/10.1016/j.jddst.2017.11.008.
DESTEFANO, Vicent; KHAN, Salaar; TABADA, Alonzo. Applications of PLA in modern medicine. Engineered Regeneration, v. 1, p. 76-87, 2020. DOI: https://doi.org/10.1016/j.engreg.2020.08.002.
FARIAS, Smirna; BOATENG, Joshua. Aspirin loaded xerogels for buccal and oral delivery for patients with dysphagia to target deep vein thrombosis. BJPharm, v. 2, n. 2, S16–S18, 2018. DOI: https://doi.org/10.5920/bjpharm.2017.18.
FRENOT, Audrey; CHRONAKIS, Ioannis S. Polymer nanofibers assembled by electrospinning. Current Opinion in Colloid & Interface Science, v. 8, n. 1, p. 64–75, 2003. DOI: https://doi.org/10.1016/S1359-0294(03)00004-9.
FRIEDL, Julian David et al. SEDDS-loaded mucoadhesive fiber patches for advanced oromucosal delivery of poorly soluble drugs. J Control Release, v. 348, p. 692–705, 2022. DOI: https://doi.org/10.1016/j.jconrel.2022.06.023.
FRIZZELL, Hannah; OHLSEN, Tiffany J.; WOODROW, Kim A. Protein-loaded emulsion electrospun fibers optimized for bioactivity retention and pH-controlled release for peroral delivery of biologic therapeutics. International Journal of Pharmaceutics, v. 533, n. 1, p. 99–110, 2017. DOI: https://doi.org/10.1016/j.ijpharm.2017.09.043.
GOLDBERG, Michael; GOMEZ-ORELLANA, Isabel. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov., v. 2, p. 289–295, 2003. DOI: https://doi.org/10.1038/nrd1067.
GUPTA, Sonal et al. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Deliv., v. 20, p. 237–246, 2013. DOI: https://doi.org/10.3109/10717544.2013.819611.
HAN, W et al. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromolecular Rapid Communications, v. 43, n. 21, 2200456, 2022. DOI: https://doi.org/10.1002/marc.202200456.
HOSSEINZADEH, Simzar et al. Predictive modeling of phenolic compound release from nanofibers of electrospun networks for application in periodontal disease. Journal of Polymer Engineering, v. 36, p. 457–464, 2016. DOI: https://doi.org.10.1515/polyeng-2015-0178.
HU, Xiuli et al. Electrospinning of polymeric nanofibers for drug delivery applications. Journal of Controlled Release, v. 185, p. 12-21, 2014. DOI: https://doi.org/10.1016/j.jconrel.2014.04.018.
IRFAN, Muhammad et al. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharmaceutical Journal, v. 24, n. 5, p. 537–546, 2016. DOI: https://doi.org/10.1016/j.jsps.2015.02.024.
JI, Wei et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res., v. 28, n. 6, p. 1259–1272, 2011. DOI: https://doi.org/10.1007/s11095-010-0320-6.
KAZSOKI, Adrienn et al. Microstructural characterization of papaverine-loaded HPC/PVA gels, films and nanofibers. European Journal of Pharmaceutical Sciences, v. 122, p. 9–12, 2018. DOI: https://doi.org/10.1016/j.ejps.2018.06.020.
KAZSOKI, Adrienn; SZABÓ, Péter; ZELKÓ, Romána. Prediction of the hydroxypropyl cellulose—poly(vinyl alcohol) ratio in aqueous solution containing papaverine hydrochloride in terms of drug loaded electrospun fiber formation. Journal of Pharmaceutical and Biomedical Analysis, v. 138, p. 357–362, 2017. DOI: https://doi.org/10.1016/j.jpba.2017.02.030.
LALIA, Boor Singh et al. A review on membrane fabrication: Structure, properties and performance relationship. Desalination, v. 326, p. 77-95, 2013. DOI: https://doi.org/10.1016/j.desal.2013.06.016.
LANNUTTI, John Joseph L. et al. Electrospinning for tissue engineering scaffolds. Materials Science and Engineering: C., v. 27, n. 3, p. 504-509, 2007. DOI: https://doi.org/10.1016/j.msec.2006.05.019.
LI, Dan; XIA, Younan. Electrospinning of Nanofibers: Reinventing the Wheel?. Advanced Materials, v. 16, n. 14, p. 1151–1170, 2004. DOI: https://doi.org/10.1002/adma.200400719.
LI, Jinghan et al. Carvedilol-loaded polyvinylpyrrolidone electrospun nanofiber film for sublingual delivery. Journal of Drug Delivery Science and Technology, v. 58, p. 101726, 2020. DOI: https://doi.org/10.1016/j.jddst.2020.101726.
MANN, Garima et al. Polymeric and electrospun patches for drug delivery through buccal route: Formulation and biointerface evaluation. Journal of Drug Delivery Science and Technology, v. 68, p. 103030, 2022. DOI: https://doi.org/10.1016/j.jddst.2021.103030.
MAŠEK, Josef et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles – important step towards effective mucosal vaccines. Journal of Controlled Release, v. 249, p. 183–195, 2017. DOI: https://doi.org/10.1016/j.jconrel.2016.07.036.
MOHAMMADINEJAD, Reza et al. Electrospun nanocarriers for delivering natural products for cancer therapy. Trends Food Sci Technol, v. 118, Part B, p. 887-904, 2021. DOI: https://doi.org/10.1016/j.tifs.2021.10.007.
MUPPALANENI, Srinath; MASTROPIETRO, David; OMIDIAN, Hossein. Mucoadhesive Drug Delivery Systems. In: BADER, Rebecca A.; PUTNAM, David A. (eds.). Engineering Polymer Systems for Improved Drug Delivery. Wiley, 2013. Chapter 10. p. 319–342. DOI: https://doi.org/10.1002/9781118747896.CH10.
NAZARI, Kazem et al. Fibrous polymeric buccal film formulation, engineering and bio-interface assessment. European Polymer Journal, v. 97, p. 147–157, 2017. DOI: https://doi.org/10.1016/j.eurpolymj.2017.09.046.
NÉMETH, Csaba et al. Fast dissolving nanofibrous matrices prepared by electrospinning of polyaspartamides. European Polymer Journal, v. 130, p. 109624, 2020. DOI: https://doi.org/10.1016/j.eurpolymj.2020.109624.
PALEM, Reddy Chinna; CHAITANYA, K. S. C; YAMSANI, Madhusudan Rao. A review on bioadhesive buccal drug delivery systems: Current status of formulation and evaluation methods. DARU, v. 19, n. 6, p. 385–403, 2011. Disponível em: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3436075/.
PORTER, Joshua R.; HENSON, Andrew; POPAT, Ketul C. Biodegradable poly(ε-caprolactone) nanowires for bone tissue engineering applications. Biomaterials, v. 30, n. 5, p. 780-788, 2009. DOI: https://doi.org/10.1016/j.biomaterials.2008.10.022.
QIN, Ze-yu et al. Fast dissolving oral films for drug delivery prepared from chitosan/pullulan electrospinning nanofibers. International Journal of Biological Macromolecules, v. 137, p. 224–231, 2019. DOI: https://doi.org/10.1016/j.ijbiomac.2019.06.224.
RASOULI, Rahimeh et al. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci, v. 19, n. 2, 1800256, 2019. DOI: https://doi.org/10.1002/mabi.201800256.
ROŠIC, Romana et al. Nanofibers and their biomedical use. Acta Pharmaceutica, v. 63, n. 3, p. 295-304, 2013. DOI: https://doi.org/10.2478/acph-2013-0024.
RUSSO, Eleonora et al. A focus on mucoadhesive polymers and their application in buccal dosage forms. Journal of Drug Delivery Science and Technology, v. 32, Part. B, p. 113-125, 2016. DOI: https://doi.org/10.1016/j.jddst.2015.06.016..
SAGITHA, P. et al. β-Cyclodextrin functionalized polyurethane nano fibrous membranes for drug delivery. Journal of Drug Delivery Science and Technology, v. 65, 102759, 2021. DOI: https://doi.org/10.1016/j.jddst.2021.102759.
SANDRI, Giuseppina et al. (Trans)buccal drug delivery. In: MARTINS, João Pedro; SANTOS, Hélder A. (eds.). Nanotechnology for Oral Drug Delivery: From Concept to Applications. Rio de Janeiro: Elsevier Inc., 2020. Chapter 8, p. 225-250. DOI: https://doi.org/10.1016/B978-0-12-818038-9.00013-2.
SELL, Scott et al. Scaffold permeability as a means to determine fiber diameter and pore size of electrospun fibrinogen. J Biomed Mater Res Part A, v. 85A, n. 1, p. 115–126, 2008. DOI: https://doi.org/10.1002/jbm.a.31556.
SHI, Wei; LU, Wensheng; JIANG, Long. The fabrication of photosensitive self-assembly au nano- particles embedded in silica nanofibres by electrospinning. J. Colloid. Interface Sci., v. 340, n. 2, p. 291–297, 2009. DOI: https://doi.org/10.1016/j.jcis.2009.09.011.
SHIRVAN, Anahita Rohani; BASHARI, Azadeh; HEMMATINEJAD, Nahid. New insight into the fabrication of smart mucoadhesive buccal patches as a novel controlled-drug delivery system. European Polymer Journal, v. 119, p. 541–550, 2019. DOI: https://doi.org/10.1016/j.eurpolymj.2019.07.010.
SILL, Travis J.; VON RECUM, Horst A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials, v. 29, n. 13, p. 1989–2006, 2008. DOI: https://doi.org/10.1016/j.biomaterials.2008.01.011.
SLAVKOVA, Marta; BREITKREUTZ, Jörg. Orodispersible drug formulations for children and elderly. European Journal of Pharmaceutical Sciences, v. 75, p. 2–9, 2015. DOI: https://doi.org/10.1016/j.ejps.2015.02.015.
SOFI, Hasham S. et al. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: Current status and future perspectives. Materials Science and Engineering C., v. 111, p. 110756, 2020. DOI: https://doi.org/10.1016/j.msec.2020.110756.
STIE, Mai Bay. et al. Mucoadhesive chitosan- and cellulose derivative-based nanofiber-on-foam-on-film system for non-invasive peptide delivery. Carbohydrate Polymers, v. 303, p. 120429, 2023. DOI: https://doi.org/10.1016/j.carbpol.2022.120429.
SULTANA, Farhana; ARAFAT, Mohamed; PATHAN, Saiful I. Preparation and evaluation of fast dissolving oral thin film of caffeine. Int. J. Pharm. Biol. Sci., v. 3, n. 1, p. 153–161, 2013. Disponível em: https://www.academia.edu/71639826/Preparation_and_Evaluation_of_Fast_Dissolving_Oral_Thin_Film_of_Caffeine.
YARIN, Alexander. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol., v. 22, n. 3, p. 310–317, 2011. DOI: https://doi.org/10.1002/pat.1781.
ZHAO, Zhiyue et al. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids and Surfaces A: Physicochemical and Engineering Aspects, v. 635, p. 128096, 2022. DOI: https://doi.org/10.1016/j.colsurfa.2021.128096.
ZUBIR, Ainaa Amirah Md et al. Electrospinning of PLA with DMF: Effect of polymer concentration on the bead diameter of the electrospun fibre. IOP Conf. Ser.: Mater. Sci. Eng., v. 778, p. 1-8, 2020. DOI: https://doi:10.1088/1757-899X/778/1/012087.
[1] PhD in Pharmacy (UFSC); Master’s degree in Pharmacy (UFSC), Specialization in Advanced Aesthetics (Faculdade Inspirar), Specialization in Hospital Infection (Veiga de Almeida University), Specialist in Oncology (SOBRAFO), Specialization in Administration of Pharmaceutical Services (UNAERP) – Degree in Pharmacy (UFRGS). ORCID: 0000-0002-3268-7738. Currículo Lattes: http://lattes.cnpq.br/1840292837167644.
[2] Advisor. Doctorate in Drugs and Medicines (USP), Master’s degree in Drugs and Medicines (USP), Graduate in Industrial Pharmacy (UFSM). ORCID: 0000-0002-7346-3693. Currículo Lattes: http://lattes.cnpq.br/3411646377586063.
[3] Post-Doctorate at UFSC and the Institut National des Sciences Appliquées de Lyon, France. PhD in Materials Science and Engineering from the Federal University of Santa Catarina, with a sandwich period at the Università degli Studi di Trento. Master’s degree in Materials Science and Engineering from the Federal University of Santa Catarina (UFSC). He holds a degree in Chemical Industrial Engineering from FEEVALE University. ORCID: 0000-0002-3086-5924. Currículo Lattes: http://lattes.cnpq.br/4597248797452360.
Enviado: 16 de agosto, 2023.
Aprovado: 24 de agosto, 2023.
















