ORIGINAL ARTICLE
MORAES, Bruna dos Santos Martins [1], CAVALCANTE, Ricardo de Sousa [2] , SILVA, Rogério Umbelino da [3], SILVA, Paulo Danilo da Silva e [4], DIAS, Claudio Alberto Gellis de Mattos [5], DENDASCK, Carla Viana [6], ARAÚJO, Maria Helena Mendonça de [7], FECURY, Amanda Alves [8]
MORAES, Bruna dos Santos Martins et al. Clinical-epidemiological profile of patients with pulmonary tuberculosis treated at a reference center in the Brazilian Amazon. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 09, Issue 07, Volume 01, pp. 27-46. July 2024. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/clinical-epidemiological-profile, DOI: 10.32749/nucleodoconhecimento.com.br/health/clinical-epidemiological-profile
ABSTRACT
Tuberculosis (TB), caused by the Mycobacterium tuberculosis complex or Koch’s bacillus, primarily occurs via inhalation when an infected individual sneezes, coughs, or speaks, releasing droplets into the air. It presents in two forms: localized only in the pulmonary parenchyma (pulmonary TB), which can manifest as primary, secondary, or miliary TB, and the form that occurs outside the pulmonary parenchyma (extrapulmonary TB). The objective of this study was to describe the clinical-epidemiological profile of patients with pulmonary tuberculosis treated at the pulmonology outpatient clinic of the Reference Center for Tropical Diseases (CRDT) in the state of Amapá, Northern Brazil, during the years 2021 and 2022. To this end, a retrospective, descriptive, and quantitative cross-sectional study was conducted, with data obtained from the pulmonology outpatient clinic of CRDT in the city of Macapá, state of Amapá, in the Brazilian Amazon. A total of 141 patients were analyzed, with 48.9% diagnosed in 2021 and 51.1% in 2022. The results of the study prompt initiatives such as research and studies to strengthen tuberculosis control and prevention strategies in health units in the state of Amapá and communities throughout Brazil, thereby improving the management of this still prevalent disease in our environment. There is also a perceived need for early recognition of symptomatic respiratory patients for timely diagnosis and treatment to reduce morbidity and mortality.
Keywords: Pulmonary Tuberculosis, Epidemiology, Clinical Profile, Koch’s Bacillus.
1. INTRODUCTION
Tuberculosis (TB), despite being one of the oldest diseases known to humanity, remains a significant public health concern as it is considered the leading cause of mortality among infectious diseases. It is an infection caused by the Mycobacterium tuberculosis complex or Koch’s bacillus. Transmission mainly occurs through inhalation when an infected individual sneezes, coughs, or talks, releasing droplets into the air (known as Pflüger droplets), which contain a bacillary load diluted in the aerosol. These droplets transform into smaller particles (Wells nuclei), containing one to two bacilli (Brasil, 2019; Teixeira et al., 2020).
Tuberculosis presents in two forms: localized only in the pulmonary parenchyma (pulmonary TB), manifesting as primary, secondary, and miliary TB, and the form that occurs outside the pulmonary parenchyma (extrapulmonary TB). Pulmonary TB is particularly significant in public health because it maintains the chain of transmission of the disease. It is transmitted in its active form through aerosol droplets expelled by the lungs and trachea. In contrast, the risk of aerosol infection is reduced in extrapulmonary TB due to its location outside the respiratory tract, with symptoms varying according to the affected system or organ (Oliveira et al., 2021; Yoshimura et al., 2021).
Pulmonary TB can be primary, occurring during the first contact with the bacillus, typically affecting children, and may present with prolonged fever, loss of appetite, and night sweats. Secondary TB, resulting from the reactivation of a latent infection, usually affects young adults and can present with pulmonary symptoms such as a cough that starts dry and progresses to productive (with purulent, mucoid, or blood-streaked sputum), chest pain, dyspnea, and in advanced cases, hemoptysis, indicating the destruction of pulmonary tissue. Systemic symptoms include evening fever, night sweats, weight loss, and asthenia (Souza; Silva, 2022; Yoshimura et al., 2021).
Within pulmonary TB, miliary TB has very specific radiological characteristics and can occur in both primary and secondary forms. It is a more severe form of the disease, with lymphatic and hematogenous dissemination, potentially affecting both the lungs and other organs. It is more common in immunosuppressed populations, especially those with severe immunocompromise. Miliary TB can present with specific symptoms depending on the affected site, such as cutaneous, neurological, cardiovascular, and even respiratory tract symptoms (Lemos et al., 2020; Tassinari et al., 2022).
Extrapulmonary tuberculosis primarily affects individuals undergoing immunosuppressive therapy and those infected with HIV (Human Immunodeficiency Virus). It represents a clinical and epidemiological challenge due to its ability to affect various organs and tissues. Consequently, it has a varied clinical presentation depending on the location, including bone pain, abdominal pain, cough, weight loss, pleural effusion, drowsiness, apathy, headache, and more. The main diagnosed forms are pleural TB (the most common form of extrapulmonary TB in HIV-negative individuals), peripheral lymph node TB, tuberculous pleural empyema, meningoencephalic TB, pericardial TB, and bone TB (Araújo et al., 2023; Yoshimura et al., 2021).
Generally, most infections will not present symptoms and are classified as latent tuberculosis, meaning that an immunocompetent individual will mount an adequate immune response through the phagocytosis of mycobacteria, which are inactivated by alveolar macrophages, followed by the activation of the adaptive immune response (Boom; Schaible; Achkar, 2021).
With the evolution of the adaptive immune response, lymphocytes and related cells migrate to the primary infection site, forming a typical granuloma where the persistent bacilli remain dormant and metabolically inactive for long periods. They can be reactivated when there is a failure in the host’s immune process, leading to disease progression (Boom; Schaible; Achkar, 2021).
The state of Amapá, one of the seven states in the northern region of Brazil, has 16 municipalities with a resident population of 733,759 people, according to the latest IBGE demographic census of 2022. Approximately 90% of its population resides in urban areas, with the majority concentrated in the metropolitan region (Macapá, Santana, Mazagão) and Oiapoque. In 2021, Amapá recorded a tuberculosis notification incidence rate (per 100,000 inhabitants) of 35.2 in 2021 and 43.1 in 2022, making it essential to evaluate the conditions leading to high rates of new cases (Brasil, 2023; IBGE, 2022).
2. OBJECTIVE
To describe the clinical-epidemiological profile of patients with pulmonary tuberculosis treated at the pulmonology outpatient clinic of the Reference Center for Tropical Diseases (CRDT) in the state of Amapá, Northern Brazil, in the years 2021 and 2022.
3. METHODOLOGY
This study is a descriptive cross-sectional study with a retrospective and quantitative approach. It is characterized this way as it analyzes and correlates the clinical and epidemiological variables of individuals along with the environment over a specified period. Additionally, it is considered quantitative because the variables were processed and quantified using statistical techniques (Dalfovo; Lana; Silveira, 2008; Lima-Costa; Barreto, 2003).
The study was conducted in the city of Macapá, in the state of Amapá. For the study’s development, data were obtained from the Pulmonology Outpatient Clinic of the Reference Center for Tropical Diseases (CRDT), which serves patients with suspected and diagnosed infectious diseases.
Patients from Macapá with pulmonary tuberculosis treated at CRDT in the years 2021 and 2022 were selected for this study. Inclusion criteria included male and female patients aged 18 and older with a confirmed diagnosis of pulmonary tuberculosis by Bacilloscopy, Rapid Molecular Test (TRM) for tuberculosis, or positive culture for mycobacteria. Exclusion criteria included patients diagnosed with tuberculosis other than the pulmonary type, those with negative results from diagnostic tests, and patients who started presumptive treatment for tuberculosis.
The instrument used for data collection was the records of patients with a diagnosis of pulmonary tuberculosis undergoing treatment during the years 2021 and 2022. After collection, the data were tabulated and processed using Microsoft Excel 365. Tables were then constructed to group the variables, which were organized into epidemiological data (age, gender, education level, and occupation) and clinical data such as signs and symptoms of tuberculosis infection, including a dry cough for more than three weeks, involuntary weight loss, daytime fever, night sweats, and other symptoms at the time of consultation.
The variables of interest were subjected to an association test, the Chi-square test. The association tests have a theoretical assumption of comparing expected and observed frequencies between qualitative (or categorical) variables to assess whether there is a relationship of dependence between them, thereby rejecting or accepting the null hypothesis that the variables of interest studied are independent. Once the Chi-square test indicates the existence of an association, Cramer’s V was also calculated to measure its degree. A p-value of less than 0.05 was considered statistically significant. For the execution of the statistical tests and descriptive analysis, the statistical software Jamovi (version 2.3) was used.
Since this research involves human subjects, it was submitted to and approved by the Ethics and Research Committee on Human Subjects of the Federal University of Amapá, in accordance with Resolution No. 466/2012 of the National Health Council, under CAAE No. 67702423.5.0000.0003. Data collection began after the letter of consent was signed by the responsible director of the Reference Center for Tropical Diseases. This study adhered to the current ethical standards for research.
4. RESULTS AND DISCUSSION
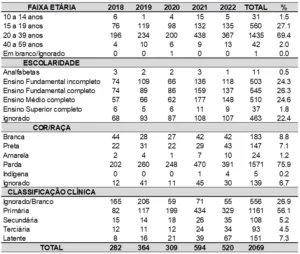
In the analysis of the data collected from the CRDT patient records, according to the inclusion criteria of this study, it was noted that there were a total of 141 patients diagnosed with pulmonary tuberculosis in 2021 and 2022. In 2021, 69 patients (48.9%) were diagnosed, and in 2022, 72 patients (51.1%).
Among the reasons for this prominence and increase are greater exposure to the disease, a high rate of HIV coinfection positivity, and interruptions in recommended treatment (Brasil, 2023). Additionally, a study conducted in the state of Amapá from 2018 to 2023 highlights another reason for this increase: improved reporting in the Notification Disease Information System (SINAN), given its coverage throughout Brazil, with reduced underreporting. The restoration of diagnostic and management flow, resumed after the pandemic period, also contributed to the increase in notifications (Cardoso et al., 2023; Silva et al., 2022).
Regarding epidemiological data, the frequency of professions showed that the most relevant groups were self-employed individuals (24.1%, n=34), domestic workers (12.1%, n=17), and healthcare professionals (6.4%, n=9), with other professions having lower percentages. However, due to incomplete records, 34.7% of patients (n=49) did not have their profession or occupation listed in the record, as shown in Table 1.
Table 1. Frequency of professions of CRDT patients diagnosed with pulmonary tuberculosis in 2021 and 2022
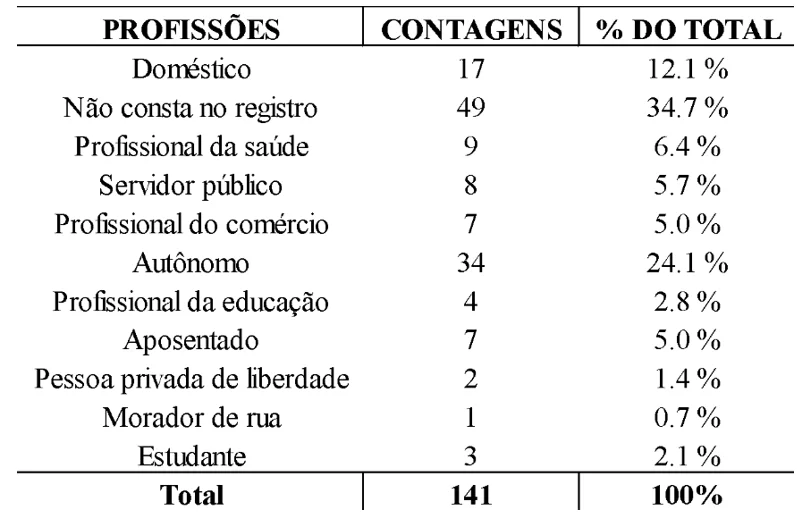
Due to their work in diverse areas and with the general public, self-employed individuals are at a higher risk of coming into contact with Koch’s bacillus. A study published in 2007 reported that independent workers have an intermediate incidence level for tuberculosis, and another study published in 2023 indicates that being self-employed is a significant predictor of a reduction in annual income by up to 20% when associated with TB, due to factors such as the lack of social security and paid sick leave (Hoshino et al., 2007; Maciel et al., 2023).
Regarding domestic workers, the data found align with studies that associate low income and unpaid occupations with increased tuberculosis incidence. Economic and social vulnerability leads to abandonment or inability to continue treatment due to circumstances such as lack of financial support for transportation to healthcare services. As for healthcare professionals, they are in closer contact with the bacillus, either through direct patient contact or through laboratory handling of contaminated biological components (Azeredo, 2019; Telarolli Junior et al., 2017).
In analyzing the gender variable, a predominance of males was observed with 66.7% (n=94), compared to females with 33.3% (n=47). This finding can be explained by the higher exposure to risk behaviors among men, neglect of their own health, delayed seeking of healthcare services, and restricted and reductionist care from the healthcare team (Siqueira et al., 2014; Tavares et al., 2020).
Regarding race, it was found that the majority, 73.8%, identified as brown (n=104), 14.9% as Black (n=21), 10.6% as White (n=15), and 0.7% as Yellow (n=1). Certain characteristics place brown and Black individuals at higher risk of illness, such as greater difficulty accessing healthcare services, lower quality of life, and experiences of intolerance and prejudice in society compared to individuals who do not self-identify as brown or Black (Tavares et al., 2020).
Regarding age, there was a close value between two age groups: 20-39 years (n=56) and 40-59 years (n=54), with an average age of 44.5 years. Considering these two age groups, the infection occurs within the economically active population, following national trends. The main factor justifying this finding is the higher exposure to the bacillus, either in the occupational area or through greater time spent outside the home. The young adult population suffers the impact of this disease on the social and economic fronts, both due to the need for job leave, reduction or loss of income, and daily costs (Brasil, 2023; Santos Junior et al., 2019).
Regarding education, it was found that 27% had incomplete elementary education, 12.1% were illiterate, 17% had completed high school, with other education levels having lower percentages. However, there was also a high rate of missing information for this variable, as shown in Table 2.
Table 2. Frequency of education levels among CRDT patients diagnosed with pulmonary tuberculosis in 2021 and 2022
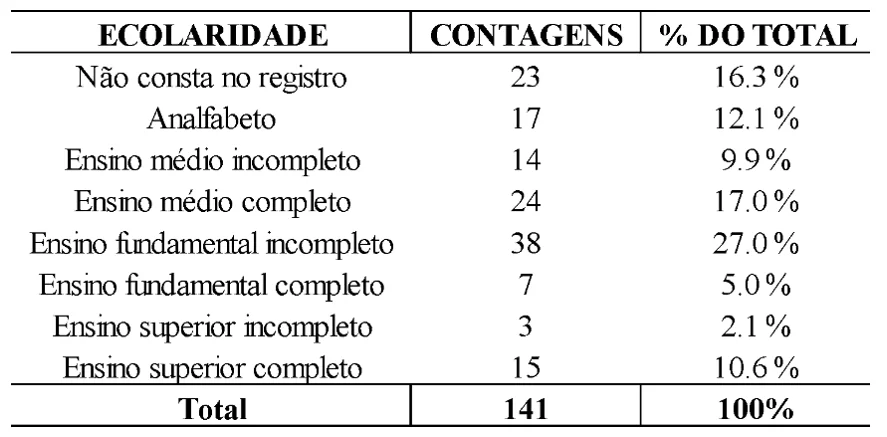
Low education is directly related to the process of illness. These individuals seem to lack a complete understanding of the severity of their disease and its progression, as well as a low awareness of the importance of seeking medical help for appropriate treatment and effective protection methods. Additionally, there is sometimes a lack of full understanding of the treatment, which hampers the elimination of Koch’s bacillus and increases the likelihood of resistance to the standard tuberculosis medication regimen (André et al., 2020; Rodrigues; Mello, 2018).
Possible factors contributing to this incompleteness of information might include a preference for recording abandonment, treatment failures, and deaths, while neglecting the collection of sociodemographic data of the patients, as well as lifestyle habits and follow-up examinations (Silva, 2023).
Regarding diagnostic tests conducted by the patients, it was found that in 2021, 20.6% underwent bacilloscopy, 38.3% underwent sputum culture, 46.8% underwent TRM-TB, and 7.1% underwent histopathology. However, in 2022, 26.2% underwent bacilloscopy, 30.5% underwent sputum culture, 49.6% underwent TRM-TB, and 3.5% underwent histopathology.
The TRM-TB test is primarily indicated for the diagnosis of pulmonary and laryngeal TB in adolescents and adults, and can also be used for extrapulmonary cases if validated biological materials are utilized. It is an accessible test available in the public health system, with results available within 2 hours and requiring only a sputum sample. Its technique detects the DNA of *M. tuberculosis* and resistance of its strains to rifampicin. Due to its greater accessibility, the high use of this test in the study population is noted (Brasil, 2019; Silva et al., 2021).
Regarding bacilloscopy, it is an important method for diagnosing TB, as it detects bacillary patients. Additionally, within the SUS (Unified Health System), it is a crucial test for the monthly monitoring of the patient’s treatment. It is a simple method with results available within 48 hours and, when performed correctly, has a sensitivity of approximately 80%. Despite the existence of faster methods, bacilloscopy is regularly used in healthcare services, as indicated by this study (Giacomet et al., 2021; Tassinari et al., 2022).
Sputum culture, although the gold standard for TB diagnosis, tends to be less utilized due to its long execution time (approximately 45 days) as it analyzes bacillus replication. It is less favored because earlier diagnosis and treatment are needed. Histopathological examination is reserved for more complex cases, where chest X-rays show extensive involvement, and for extrapulmonary cases (Giacomet et al., 2021).
Regarding clinical data, the results showed a high prevalence of systemic changes, with cough being the most common symptom, occurring in about 56.7% of the cases (80 patients), followed by weight loss at 40.4% (57 patients), and daytime fever at 37.6% (53 patients) of the total. Symptoms such as night sweats, dyspnea, chest pain, and hemoptysis had less significant percentages, each occurring in less than 15% of the cases. Hemoptysis was present in only 5% of the cases, as shown in Table 3.
The predominance of cough as a symptom is characteristic of the disease, justified by the involvement of Koch’s bacillus in the pulmonary parenchyma, which triggers an immune response leading to inflammatory response and tissue damage, thereby activating cough receptors in the respiratory tract (De Souza Tej et al., 2023).
Weight loss is associated with loss of appetite and decreased intake due to cough-related discomfort. The lower occurrence of fever and night sweats is explained by the host’s immune factors, which involve the release of a certain amount of pro-inflammatory substances and cytokines when in contact with mycobacteria and their antigens. As for the occurrence of other symptoms (chest pain, dyspnea, and hemoptysis), these are associated with more advanced clinical stages, which, in the present study, were observed to be the minority at the time of diagnosis (Santana; Seniski, 2016; Alves et al., 2022).
Table 3. Frequency of Symptoms Among CRDT Patients Diagnosed with Pulmonary Tuberculosis in 2021 and 2022
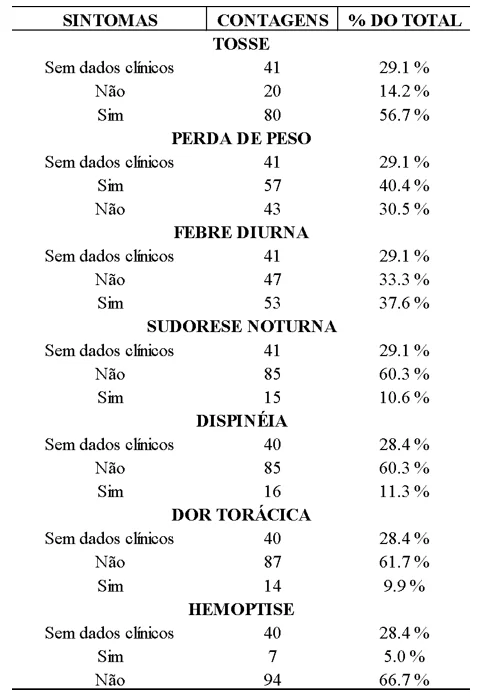
It is noteworthy that of the reports analyzed, approximately 28.4% (n=40) did not include clinical data.
This lack of data may be related to manual entry by professionals, the opening of new records, lack of chronological order, division by specialties, and the absence of technology for grouping and standardizing information. This results in lost records or gaps in patient registration and history (Freitas et al., 2022).
Additionally, other symptoms (asthenia and loss of appetite, constant vomiting, anorexia, abdominal pain, hoarseness, and back pain) account for 5.6% of the total symptoms (n=8), as shown in Table 4.
Table 4. Frequency of Other Symptoms Among CRDT Patients Diagnosed with Pulmonary Tuberculosis in 2021 and 2022
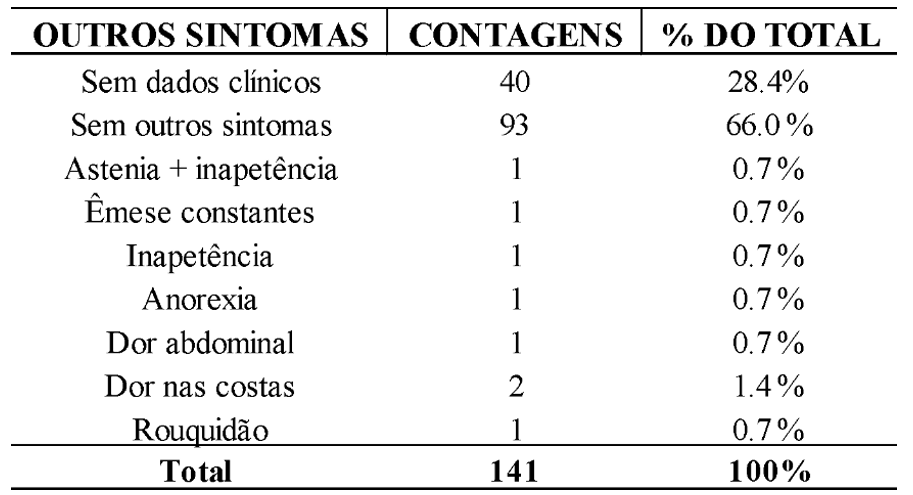
The disparity in the occurrence of these symptoms compared to the more prevalent ones is explained by other studies due to variations in each individual’s immune response, the natural history of the disease, and individual factors such as age and underlying medical conditions (Kendall et al., 2021; Rodríguez et al., 2018).
Regarding the analyzed data and the correct completion of records, the completeness of clinical and epidemiological data was considered. It was observed that 51.7% of the records had complete data, 22.7% had missing clinical data and partial epidemiological data, 18.4% had complete clinical data and partial epidemiological data, and 7.1% had no clinical data.
It was noted that in both the clinical and epidemiological groups, some variables were either not filled out or were filled out partially. As the notification is manual, there is a reliance on a professional to enter data into the notification system or complete the notification and registration forms. If this professional is not trained for such tasks, there is a possibility of gaps due to lack of patient information, as occurred in this study (Tomberg et al., 2019). The consequence is a distorted number of notifications, which directly impacts the actual number of infected individuals, their treatment and follow-up, as well as a higher exposure of people to this condition. Furthermore, incomplete data affects the quality of the service and epidemiological surveillance, as it is the initial step for taking control and management actions in a given region (Costa et al., 2023).
Statistical association calculations between clinical and epidemiological variables were performed, with the chi-square test of independence showing an association between the performance of the Mycobacterium Culture and the year it was conducted (X²(1)= 5.64; p=0.018; Cramer’s V=0.20), as well as between the presence of chest pain and age group (X²(6)= 12.9; p=0.044; Cramer’s V=0.214). Other analyses did not show statistical significance, as can be seen in Table 5.
Table 5. Results of the association tests and their respective p-values
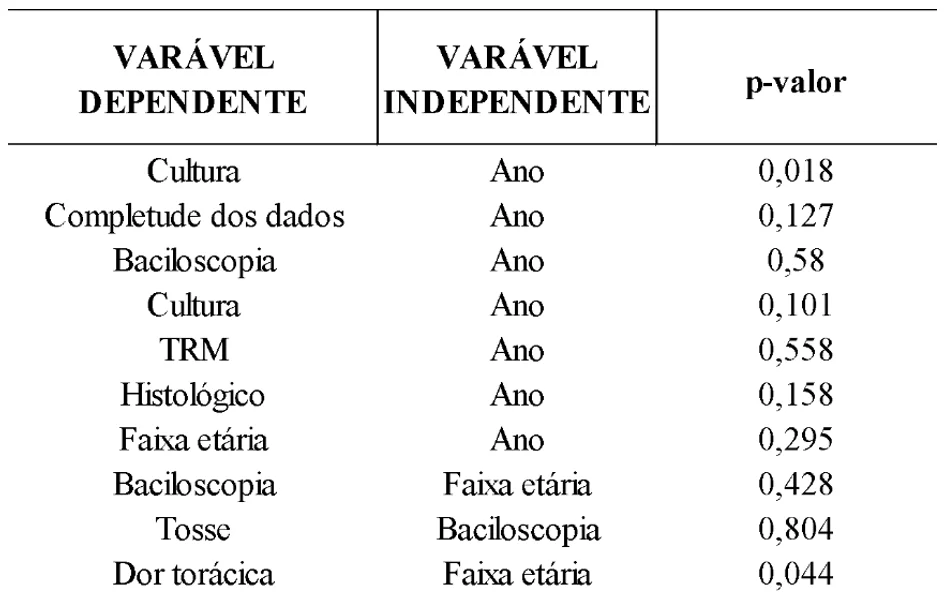
Regarding the variables that showed significance (p < 0.05), according to the literature, the association between chest pain and age group indicates that young adults are more likely to experience extrapulmonary tuberculosis, particularly pleuritic TB, though this is not a strict rule. In elderly individuals, a higher percentage is observed due to associated pre-existing comorbidities and lifestyle habits related to smoking. Thus, the association between chest pain and age group does not point to a mandatory occurrence but rather a possible scenario (Chaves et al., 2017; Silva et al., 2021).
Regarding culture and the year it was performed, there are no other studies that have observed such an association. However, as the gold standard test for diagnosing pulmonary TB, when accessible and performed, its sensitivity is around 80% and its specificity is 98%. Thus, there is a direct relationship between proper execution and positivity (if infection is present), as highlighted by the present study (Silva et al., 2021).
5. CONCLUSIONS
The results of this study highlight the need for initiatives such as research and studies to strengthen tuberculosis control and prevention strategies in health units in the state of Amapá and across communities in Brazil. This is crucial for improving the management of this still prevalent disease. There is also a need for early recognition of symptomatic respiratory patients for timely diagnosis and treatment to reduce morbidity and mortality.
It is also crucial to emphasize the importance of accurate data entry in records that are already standardized by the Ministry of Health, particularly concerning profession, comorbidities, and clinical data such as signs and symptoms of these patients. Proper data collection contributes to improving the quality of information, which in turn supports health authorities in implementing public policies aimed at reducing or eradicating the disease in the country.
REFERENCES
ALVES, A. C. F. P. B. et al. Tuberculosis immunology: a narrative literature review. Arquivos de Asmas Alergia e Imunologia, [s. l.], v. 6, n. 2, p. 239–250, 2022. DOI: http://dx.doi.org/10.5935/2526-5393.20220024.
ANDRÉ, S. R. et al. Tuberculosis associated with the living conditions in an endemic municipality in the North of Brazil*. Revista Latino-Americana de Enfermagem, [s. l.], v. 28, p. e3343, 2020. DOI: https://doi.org/10.1590/1518-8345.3223.3343.
ARAÚJO, F. G. A. de et al. Tuberculose extrapulmonar com envolvimento renal e intestinal: Um desafio no diagnóstico precoce. Research, Society and Development, [s. l.], v. 12, n. 14, p. e80121444516–e80121444516, 2023. DOI: http://dx.doi.org/10.33448/rsd-v12i14.44516.
AZEREDO, A. C. V. Tuberculose em profissionais da saúde e o impacto da implantação de medidas de controle de infecção. Porto Alegre: 2019. Disponível em: https://lume.ufrgs.br/handle/10183/205993. Acesso em: 23 mar. 2024.
BOOM, W. H.; SCHAIBLE, U. E.; ACHKAR, J. M. The knowns and unknowns of latent Mycobacterium tuberculosis infection. The Journal of Clinical Investigation, [s. l.], v. 131, n. 3, p. e136222, 136222, 2021. DOI: https://doi.org/10.1172/JCI136222.
BRASIL. Ministério da Saúde. Secretaria de Vigilância em Saúde e Ambiente. Boletim Epidemiológico de Tuberculose – Número Especial | MAR. 2023. Brasília: Ministério da Saúde, 2023. Disponível em: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2023/boletim-epidemiologico-de-tuberculose-numero-especial-mar.2023/view. Acesso em: 19 out. 2023.
BRASIL. Ministério da Saúde. Secretaria de vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis. Manual de recomendações para o controle da tuberculose no Brasil. 2 edição. Brasília: Ministério da Saúde, 2019. Disponível em: https://bvsms.saude.gov.br/bvs/publicacoes/manual_recomendacoes_controle_tuberculose_brasil_2_ed.pdf. Acesso em: 24 mar. 2024.
CARDOSO, R. F. et al. Aspectos clínicos e epidemiológicos da tuberculose no estado do Amapá. Brazilian Journal of Health Review, [S. l.], v. 6, n. 3, p. 12689-12703, 14 jun. 2023. DOI 10.34119/bjhrv6n3-333. DOI: https://doi.org/10.34119/bjhrv6n3-333.
COSTA, G. F. et al. Factors Associated with Tuberculosis Outcome in a Hyperendemic City in the North of Brazil. Healthcare (Basel, Switzerland), [s. l.], v. 11, n. 4, p. 508, 2023. DOI: https://doi.org/10.3390%2Fhealthcare11040508.
CHAVES, E. C. et al. Epidemiological, clinical and evolutionary aspects of tuberculosis among elderly patients of a university hospital in Belém, Pará. Revista Brasileira de Geriatria e Gerontologia, [s. l.], v. 20, n. 1, p. 45–55, 2017. DOI: https://doi.org/10.1590/1981-22562017020.160069.
DALFOVO, M. S.; LANA, R. A.; SILVEIRA, A. Métodos Quantitativos e Qualitativos: Um Resgate Teórico. Revista Interdisciplinar Científica Aplicada. Blumenau, v. 2, n. 4, p. 1–13, 2008. Disponível em: https://portaldeperiodicos.animaeducacao.com.br/index.php/rica/article/view/17591/11376. Acesso em 20 mar. 2024.
DE SOUZA TEJ, R. I. et al. Aspectos Clínicos, Epidemiológicos e Laboratoriais de Portadores de Tuberculose Pulmonar e Extrapulmonar Provenientes de Serviços Públicos do Estado de Pernambuco. The Brazilian Journal of Infectious Diseases, [s. l.], v. 27, p. 103607, 2023. DOI: https://doi.org/10.1016/j.bjid.2023.103607.
FREITAS, B. S. et al. Manejo da tuberculose pulmonar na atenção primária à saúde de um município do Tocantins: Uma análise documental. Revista Ciência e Estudos Acadêmicos de Medicina, [s. l.], v. 16, n. 2, p. 35–54, 2022. Disponível em: https://periodicos.unemat.br/index.php/revistamedicina/article/view/6544/7350. Acesso em: 29 mar. 2024.
GIACOMET, C. L. et al. Tendência temporal da incidência de tuberculose e sua distribuição espacial em Macapá-AP. Revista de Saúde Pública, [s. l.], v. 55, p. 96, 2021. DOI: https://doi.org/10.11606/s1518-8787.2021055003431.
HOSHINO, H. et al. Estimation of TB incidence by labor status. Kekkaku: [Tuberculosis], [s. l.], v. 82, n. 9, p. 685–695, 2007. DOI:10.11400/KEKKAKU1923.82.685.
IBGE. Cidades. Amapá. Panorama. [S. l.], 19 de mar. de 2022. Disponível em: https://cidades.ibge.gov.br/brasil/ap/panorama. Acesso em: 24 mar. 2024.
KENDALL, E. A. et al. The Spectrum of Tuberculosis Disease in an Urban Ugandan Community and Its Health Facilities. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, [s. l.], v. 72, n. 12, p. e1035–e1043, 2021. DOI: https://doi.org/10.1093/cid/ciaa1824.
LEMOS, V. de S. et al. Tuberculose miliar em paciente imunocompetente – Relato de caso/Miliary tuberculosis in an immunocompetent patient – Case report. Brazilian Journal of Health Review, [s. l.], v. 3, n. 6, p. 17226–17234, 2020. DOI: https://doi.org/10.34119/bjhrv3n6-143.
LIMA-COSTA, M. F.; BARRETO, S. M. Tipos de estudos epidemiológicos: conceitos básicos e aplicações na área do envelhecimento. Epidemiologia e Serviços de Saúde, [s. l.], v. 12, n. 4, 2003. DOI: http://dx.doi.org/10.5123/S1679-49742003000400003.
MACIEL, E. L. N. et al. The economic burden of households affected by tuberculosis in brazil: first national survey results, 2019-2021. PLOS ONE. [S. l.], 2023. DOI: https://doi.org/10.1371/journal.pone.0287961.
OLIVEIRA, G. do C. A. et al. Epidemiological profile of the population with tuberculosis in the Rio de Janeiro State / Perfil epidemiológico da população com tuberculose no Estado do Rio de Janeiro. Revista de Pesquisa Cuidado é Fundamental Online, [s. l.], v. 13, p. 197–204, 2021. DOI: https://doi.org/10.9789/2175-5361.rpcfo.v13.8211.
RODRIGUES, M. W.; MELLO, A. G. N. C. Tuberculose e escolaridade: Uma revisão da literatura. Revista Internacional de apoyo a la inclusión, logopedia, sociedad y multiculturalidad, [s. l.], v. 4, n. 2, 2018. DOI: https://doi.org/10.17561/riai.v4.n2.1.
RODRÍGUEZ, N. Á. et al. Caracterización clínico-epidemiológica de pacientes con tuberculosis en el municipio Cumanayagua. Provincia Cienfuegos. 2007-2017/Clinical-epidemiological characterization of tuberculosis in the Cumanayagua Municiality. Cienfuegos Province. 2007-2017. [s. l.], 2018. Disponível em: http://scielo.sld.cu/pdf/ms/v16n5/ms06516.pdf. Acesso em: 25 mar. 2024.
SANTANA, J. E. N.; SENISKI, G. G. Estado Nutricional dos pacientes casos novos de tuberculose pulmonar bacilífera no município de Pinhais- PR. Anais do EVINCI – UniBrasil, [s. l.], v. 2, n. 1, p. 316–316, 2016. Disponível em: https://portaldeperiodicos.unibrasil.com.br/index.php/anaisevinci/article/view/1845. Acesso em: 29 mar. 2024.
SANTOS JUNIOR, C. J. et al. Aspectos clínicos e epidemiológicos da tuberculose em pacientes com HIV/aids. Medicina (Ribeirão Preto), [s. l.], 2019. DOI: https://doi.org/10.11606/issn.2176-7262.v52i3p231-238.
SILVA, D. R. et al. Consenso sobre o diagnóstico da tuberculose da Sociedade Brasileira de Pneumologia e Tisiologia. Jornal Brasileiro de Pneumologia, [s. l.], v. 47, p. e20210054, 2021. DOI: https://doi.org/10.36416/1806-3756/e20210054.
SILVA, L. M. et al. O cenário da Tuberculose no Brasil: impactos da pandemia da COVID-19 na subnotificação e descontinuidade do tratamento/ The Tuberculosis scenario in Brazil: impacts of the COVID-19 pandemic on unreporting and discontinuity of treatment. Brazilian Journal of Health Review, [s. l.], v. 5, n. 5, p. 21067–21081, 2022. DOI: https://doi.org/10.34119/bjhrv5n5-260.
SILVA, M. S. Completude dos Dados de Tuberculose no Sistema de Informação em Saúde – uma Revisão Integrativa. Foz do Iguaçu, 2023. Disponível em: https://dspace.unila.edu.br/handle/123456789/7512. Acesso em: 29 mar. 2024.
SIQUEIRA, B. P. D. J. et al. Men and health care in the social representations of health professionals. Escola Anna Nery – Revista de Enfermagem, [s. l.], v. 18, n. 4, 2014. DOI: DOI: 10.5935/1414-8145.20140098.
SOUZA, D. S.; SILVA, C. S. Avaliação de casos de Tuberculose em uma capital da Amazônia Ocidental Brasileira de 2011 a 2020. REVISTA CEREUS, [s. l.], v. 14, n. 3, p. 245–257, 2022. DOI: https://doi.org/10.18605/2175-7275/cereus.v14n3p245-257.
TASSINARI, E. R. et al. Métodos diagnósticos para tuberculose: uma revisão integrativa. BioSCIENCE, [s. l.], v. 80, n. S1, p. 8–8, 2022. DOI: https://doi.org/10.55684/80.S1.8.
TAVARES, C. M. et al. Tendência e caracterização epidemiológica da tuberculose em Alagoas, 2007-2016. Cadernos Saúde Coletiva, [s. l.], v. 28, p. 107–115, 2020. DOI: https://doi.org/10.1590/1414-462X202028010381.
TEIXEIRA, A. Q. et al. Tuberculose: conhecimento e adesão às medidas profiláticas em indivíduos contatos da cidade do Recife, Pernambuco, Brasil. Cadernos Saúde Coletiva, [s. l.], v. 28, p. 116–129, 2020. DOI: https://doi.org/10.1590/1414-462X202028010332.
TELAROLLI JUNIOR, R. et al. Clinical and epidemiological profile of tuberculosis in an urban area with high human development index in southeastern Brazil. Time series study. Sao Paulo Medical Journal, [s. l.], v. 135, p. 413–419, 2017. DOI: https://doi.org/10.1590/1516-3180.2016.0260210317.
TOMBERG, J. O. et al. Registros na detecção da tuberculose: percepção dos profissionais de saúde. [S. l.], p. 1-7, 2019. DOI: 10.1590/2177-9465-EAN-2019-0008.
YOSHIMURA, F. K. et al. Tuberculose: revisão de literatura/ Tuberculosis: a review of the literature. Brazilian Journal of Health Review, [s. l.], v. 4, n. 3, p. 14223–14231, 2021. DOI: https://doi.org/10.34119/bjhrv4n3-354.
NOTE
The authors used ChatGPT version 04 for grammatical, orthographic, and verbal agreement corrections. However, all content searches and article quality assessments were conducted independently by the authors.
[1] Medical student (UNIFAP). ORCID: https://orcid.org/0009-0002-3818-8005 Currículo Lattes: https://lattes.cnpq.br/3509135412458006.
[2] Medical student (UNIFAP). ORCID: https://orcid.org/0009-0005-0931-457X. Currículo Lattes: http://lattes.cnpq.br/8837742795293619.
[3] Medical student (UNIFAP). ORCID: https://orcid.org/0009-0002-9543-142X. Currículo Lattes: http://lattes.cnpq.br/7491363722883977.
[4] Bachelor’s degree in Medicine (UEPA). Residency in Pulmonology (HUJBB). ORCID: https://orcid.org/0009-0005-4366-432X. Currículo Lattes: http://lattes.cnpq.br/8194220871551027.
[5] Biologist, Ph.D. in Theory and Research of Behavior (UFPA), Professor and Researcher at the Institute of Basic, Technical, and Technological Education of Amapá (IFAP), in the Professional and Technological Education Graduate Program (PROFEPT IFAP), and in the Biodiversity and Biotechnology of the Legal Amazon Graduate Program (Rede BIONORTE – Amapá Hub – UNIFAP). ORCID: https://orcid.org/0000-0003-0840-6307. Currículo Lattes: http://lattes.cnpq.br/8303202339219096.
[6] Ph.D. in Communication and Semiotics from the Pontifical Catholic University of São Paulo (PUC/SP). Ph.D. in Clinical Psychology and Psychoanalysis. Master’s in Religious Sciences from Mackenzie Presbyterian University. Master’s in Clinical Psychoanalysis. Bachelor’s in Biological Sciences. Bachelor’s in Theology. Has over 15 years of experience with Scientific Methodology (Research Method) in guiding scientific production for Master’s and Doctoral students. Specialist in Market Research and Research related to the Health field. ORCID: https://orcid.org/0000-0003-2952-4337. Lattes: https://lattes.cnpq.br/2008995647080248.
[7] Ph.D. candidate in Health Care Sciences at Federal Fluminense University (UFF). Master’s in Health Sciences from Federal University of São Paulo (UNIFESP); Specialist in Family Health from State University of Ceará (UECE), Brazil; Specialist in Occupational Medicine from Gama Filho University (UGF), Brazil; Specialist in Occupational Health and Human Ecology from Oswaldo Cruz Foundation (FIOCRUZ), Brazil; Specialist in Internal Medicine from the Hospital dos Servidores do Estado do Pará; Medical doctor from State University of Pará (UEAP). ORCID: 0000-0002-7742-144X. Currículo Lattes: http://lattes.cnpq.br/8427706088023830.
[8] Biomedical Scientist, Ph.D. in Tropical Diseases (UFPA), Professor and researcher at the Medical School of the Macapá Campus, Federal University of Amapá (UNIFAP), in the Graduate Program in Health Sciences (PPGCS/UNIFAP), and the Graduate Program in Science and Mathematics Education (RedECIM). ORCID: https://orcid.org/0000-0001-5128-8903 Currículo Lattes: http://lattes.cnpq.br/9314252766209613.
Material received: April 30, 2024.
Peer-reviewed: July 1, 2024.
Edited material approved by authors: July 5, 2024.