ORIGINAL PAPER
RODRIGUES, Camila Juliano Salvador [1], SILVEIRA, Elita Ferreira da [2], VARGAS, Rafael da Silveira [3], GIACOMO, Giordano Gatti de [4], BIANCHIN, Marino Muxfeldt [5]
RODRIGUES, Camila Juliano Salvador. Et al. Cancer stem cells markers associated with development and aggressive phenotype in pancreatic cancer. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 10, Vol. 12, pp. 102-122. October 2020. ISSN:2448-0959, Access link in: https://www.nucleodoconhecimento.com.br/health/pancreatic-cancer, DOI: 10.32749nucleodoconhecimento.com.br/health/pancreatic-cancer
ABSTRACT
Background: Cancer stem cells, also known as tumor-initiating cells, are suggested to be responsible for drug resistance and cancer development due in part to their ability to self-renew themselves and differentiate into heterogeneous lineages of cancer cells. Objective: This study was designed to investigate the role of cancer stem cells in pancreatic cancer. Methods: A retrospective clinicopathological analysis was undertaken in 112 patients diagnosed with pancreatic ductal adenocarcinoma between 2005 and 2010, and immunohistochemistry was performed with antibodies against CD133, CD24, and OCT4. Positive nuclear, cytoplasmic or membrane staining for each antibody was rated on staining intensity, being classified into low/moderate or strong staining groups. Results were analyzed relative to each patient’s clinicopathological parameters. Results: There was an established relationship between the staining of the markers with some variables associated with worse prognosis, being the three markers present in most tumor cells and associated with tumor progression. We suppose that cancer stem cells are present from the beginning of tumor initiation and are intrinsically related to tumor development. Although the presence of stem cells has been associated with molecular biology of various tumors, their expression in pancreatic cancer has not yet been clinically reported. Conclusion: The presence of stem cells and their role in pancreatic cancer tumorigenesis may be considered as valuable prognostic factors, although the mechanism involved needs further investigation. Increasing insights into role of cancer stem cells and carcinogenesis can ultimately generate new ideas for molecularly based diagnostic and therapeutic approaches.
Keywords: Cancer stem cells, pancreatic cancer, AC133 Antigen, CD24 Antigen, OCT4.
INTRODUCTION
Despite progress in cancer diagnosis and treatment over the past decades, it remains one of the most common causes of death worldwide. While potentially curable when diagnosed early, early diagnosis remains a challenge in daily clinical practice. Many cancers are diagnosed at a late stage, when therapeutic options are no longer effective (BARBOSA et al., 2018).
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive disease usually diagnosed in an advanced stage and for which few effective therapies are available. It is a neoplasm whose incidence is almost equal to its mortality. The reason why pancreatic cancer has such a poor prognosis and why it has such a low therapeutic response to chemotherapy and radiotherapy are questions that have motivated the search for an answer that can modify the course of this disease. Despite advances in surgical techniques, which today are considered the only curative option, the average 5-year survival rate is less than 5% (1-3%) (BARBOSA et al., 2018; SOTO et al., 2006). It is considered the neoplasia with the worst prognosis out of more than 60 types of cancers, a fact evidenced by its incidence rate almost equaling the mortality rate (SAKORAFAS et al., 2000). It should be noted that early cancer detection, at a stage that can still be cured, is associated with greater therapeutic effectiveness and is currently one of the ways to improve the outcome of patients diagnosed with pancreatic cancer (ALLEGRA; TRAPASSO, 2012).
The molecular biology of pancreatic cancer is directly related to its rapid progression and low therapeutic response. It is well-documented that the appearance of a tumor cell is the result of an accumulation of mutations in its DNA capable of morphologically and functionally altering its normal properties (FANG et al., 2013; RICCI-VITIANI et al., 2007). The triggering of this process and its molecular evolution, culminating in the formation of the tumor cell and its pertinent characteristics, characterize the process of carcinogenesis. It is well-understood in the literature that the development of a neoplasia involves a set of molecular changes that lead not only to the emergence and survival of a tumor cell, but to its ability to invade adjacent tissues and form distant metastases (LI; JIAO, 2003).
Among the processes most recently implicated in carcinogenesis and tumor progression, changes in cancer stem cells (CSCs) are still poorly studied in pancreatic cancer with no satisfactory results, and they are deserving of greater efforts in elucidating their effects and relationship in acquiring a pancreatic malignant phenotype. Recent evidence has suggested that CSCs play a crucial role not only in the generation and maintenance of different tissues, but also in the development, progression, and therapeutic resistance of different tumor types (BALIC et al., 2012).
CSCs belong to a differentiated group of cells within a tumor with self-renewing and differentiating properties, similar to normal stem cells, giving rise to heterogeneous tumor cell lines that form one tumor. They can divide and originate several cells that make up tumors, thus perpetuating their growth and offering resistance to chemotherapy by mechanisms not yet well-understood. Because of this, they represent an important research target (TAI, 2005).
The intrinsic aggressiveness of pancreatic cancer, with its high capacity for local invasion, high metastatic potential, late diagnosis, and therapeutic resistance, seems to be partly related to the population of CSCs that make up these tumors (HU; FU, 2012). Furthermore, it has been shown that PDAC contains not only a homogeneous population of CSCs, but diverse subpopulations that may be involved in tumor progression. Until now, identified pancreatic CSCs constitute a minority of tumor component cells (about 1-5%), and have a high capacity for self-renewal (ANSARI et al., 2012). The most important clinical aspect, however, is that these CSCs are highly resistant to chemotherapy and radiotherapy, resulting in resistance and rapid recurrence of the disease. Currently, one of the great challenges of oncology is identifying and studying the characteristics of these CSCs, especially in PDAC, in which few discoveries have been made (KOBAYASHI; NORONHA, 2015).
MATERIALS AND METHODS
CASE SELECTION AND TUMOR SAMPLES
The tissue samples were from a cohort of 112 consecutive pancreatic cancers diagnosed from 2005 to 2010, selected from the routine care of Hospital de Clínicas de Porto Alegre (HCPA), by the Department of Pathology and Group of Biliary Tract and Pancreas. Immunohistochemical analyses were performed in paraffin-embedded tissue blocks already stored in the Department of Pathology of HCPA, after approval by the Scientific Committee and the Committee on Ethics in Health Research of the institution. The following variables were recorded from medical records: age, sex, tumor grade, lymph node status, occurrence of metastasis, treatment received (type of surgery, radiation therapy, and chemotherapy), disease free-survival, and overall survival.
TISSUE MICROARRAY (TMA) IMMUNOHISTOCHEMICAL ANALYSIS
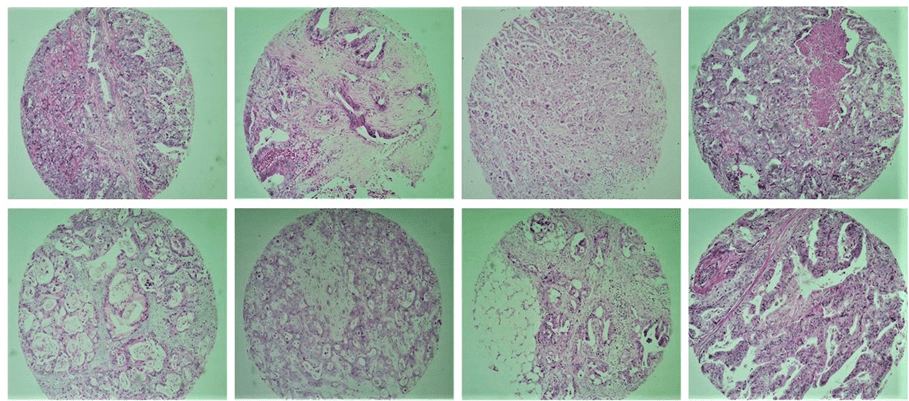
Tissue Microarray (TMA) array is a technique first described by Kononen et al. (1998) and is a great tool for investigative pathology. It is about grouping in a single block a reasonable number of tissue samples, thus allowing the study of immunohistochemical expression of molecular markers, with great use of the material, presence of internal control, and time and cost effective. This technique also allows the standardization of reactions, efficient representativeness through the use of different areas of the tumor, study of large numbers of samples simultaneously, ease of interpretation and large-scale research of prognostic or predictive factors of cancer (Figure 1).
Fig. 1. Exemplifies some tumor tissue samples included in the study by the TMA technique
STATISTICAL ANALYSIS
To verify the association between immunohistochemical variables and immunoreaction intensity with the characterization variables of individuals, a non-parametric approach was used with Fisher’s exact test. To verify the association of the numerical variable age with immunohistochemical variables and intensity, the Mann-Whitney U test was used.
The association between stem cell markers and different clinicopathological characteristics of patients [including age, sex, tumor differentiation, stage, Tumor-Node-Metastasis (TNM) category, and tumor resection] was evaluated by Pearson’s correlation coefficient or χ2 tests as appropriate. For intensity, the categories were dichotomized into low/moderate intensity (0-2+) or high intensity (≥3+).
The Kaplan-Meier method was used for estimating the probability of survival. Survival curves were compared using the log-rank test, which asserts under the null hypothesis that survival functions do not differ from each other.
Overall survival time was measured from the time of surgery or biopsy to either the date of death (as a result of any cause) or to the last follow-up. Disease-free survival time was measured from the date of histological diagnosis or surgery to the date of the first disease-free failure event (defined as locoregional relapse), distant disease, or death as a result of any cause. Significance was established at P < 0.05. All statistical analyses were performed using R (version 3.6.0).
RESULTS
Immunohistochemical analyses indicated the presence of tumor stem cell surface markers for the three antibodies analyzed in PDAC cells. A total of 112 patients with pancreatic cancer were included in this study: 59 (52.6%) males and 53 (47.4%) females, with a median age at diagnosis of 63 years (range, 36-93 years, SD ± 10.3) and a median post-diagnosis follow-up period of 16.2 months.
There was no significant difference between sexes when comparing antibody signal intensity. Fifty patients (48.08%) died during the follow-up period.
Among all patients included, 16 (15.2%) were in pathologic stage I, 32 (30.4%) were in pathologic stage II, 25 (23.8%) were in pathologic stage III, and 32 (30.4%) were in pathologic stage IV. In cases where information on tumor grade was available, 2.8% of carcinomas were well-differentiated, 62.8% were moderately differentiated, and 34.2% were poorly differentiated.
Regarding the TNM stage, 11.2% were T1 (tumor smaller than 2 cm), 38.3% were T2 (tumor larger than 2 cm or invading neighboring tissues), 19.6% were T3 (tumor of any size invading nearby tissues, but without compromising the celiac plexus or superior mesenteric artery), 21.5% were T4 (tumor of any size compromising vital organs and celiac plexus or superior mesenteric artery), and 9.3% were Tx (extent of the tumor is unknown). For lymph node involvement, 31.7% were in stage N0 (no lymph node metastasis), 31.7% were N1 (regional lymph node metastasis), 6.5% were N2 (more than one affected lymph node chain), and 29.9% were Nx (not evaluable). Regarding the presence of metastasis, 66.6% were in stage M0 (no distant metastases), 30.2% were M1 (distant metastasis), and 3.6% were Mx (not evaluable).
About 75.9% of samples were obtained from pancreatic tumor tissue, and about 26% were from metastasis samples, with the liver (65.7%) being the most affected site, followed by the lymph nodes (14.2%), peritoneum (8.5%), and others (11.4%).
Palliative treatment was given to 68 individuals, or 76.40% of the total number of people analyzed.
Regarding the location of the tumor, 81.6% of tumors were located in the head of the pancreas, 22.9% in the body, and 6.4% in the tail.
Table 1 summarizes the general descriptive analysis of the individuals involved in the study.
Table 1. General Descriptive Analysis
| Variables | N | % |
| Sex | ||
| Female | 53 | 47.4 |
| Male | 59 | 52.6 |
| Age | ||
| 35-59 years | 37 | 33.9 |
| 60-69 years | 39 | 35.8 |
| 70-93 years | 33 | 30.3 |
| Death (n=104) | ||
| No | 54 | 51.9 |
| Yes | 50 | 48.1 |
| Surgical treatment (n=108) | ||
| Palliative | 42 | 38.9 |
| Partial surgery | 19 | 17.6 |
| DPT# | 47 | 43.5 |
| Surgical treatment (n=89) | ||
| No | 21 | 23.6 |
| Yes | 68 | 76.4 |
| Tumor differentiation (n = 105) | ||
| Well differentiated | 3 | 2.9 |
| Moderate differentiated | 66 | 62.8 |
| Poor differentiated | 36 | 34.3 |
| Specimen/Sample (n=108) | ||
| Metastasis | 26 | 24.1 |
| Pancreas | 82 | 75.9 |
| Tumor pathologic stage T (n=107) | ||
| T1 | 12 | 11.2 |
| T2 | 41 | 38.3 |
| T3 | 21 | 19.6 |
| T4 | 23 | 21.5 |
| TX | 10 | 9.4 |
| Tumor pathologic stage N (n= 107) | ||
| N0 | 34 | 31.8 |
| N1 | 34 | 31.8 |
| N2 | 7 | 6.5 |
| NX | 32 | 29.9 |
| Tumor pathologic stage M (n = 109) | ||
| M0 | 72 | 66.1 |
| M1 | 33 | 30.2 |
| MX | 4 | 3.7 |
| Tumor pathologic general stage (n=105) | ||
| 1 | 16 | 15.2 |
| 2 | 32 | 30.5 |
| 3 | 25 | 23.8 |
| 4 | 32 | 30.5 |
| MTX (n=35) | ||
| Liver | 23 | 65.7 |
| Lymph Nodes | 5 | 14.3 |
| Peritoneum | 3 | 8.6 |
| Others | 4 | 11.4 |
| Location: Tumor Head | ||
| Yes | 89 | 81.7 |
| Location: Tumor Tail | ||
| Yes | 7 | 6.4 |
| Location: Tumor Body | ||
| Yes | 25 | 22.9 |
| Total | 112 | 100.0 |
#DPT: Duodenopancreatectomy
CD133 IMMUNOSTAINING IN CANCER STEM CELLS IN PANCREATIC DUCTAL ADENOCARCINOMA CELLS
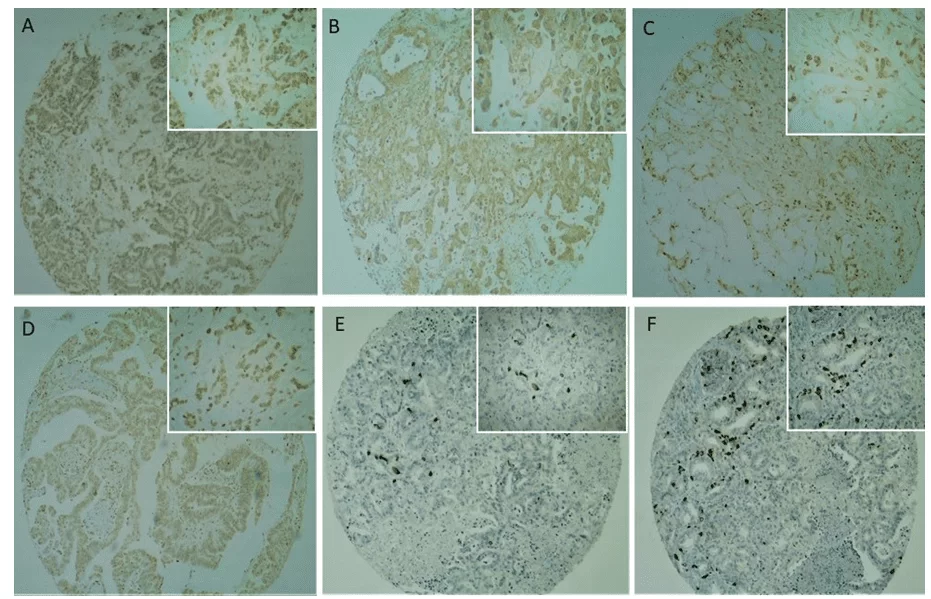
CD133 immunostaining was present in the plasma membrane and cytoplasm at moderate to strong intensity (Figures 2C and 2D), with a predominantly strong intensity. A positive or negative reaction was confirmed with the positive control (placenta) used in the study. No CD133 immunoreactivity was observed in normal pancreatic ductal epithelium (Figure 2).
Fig. 2. Immunohistochemical analysis of Cancer stem cells markers in TMA pancreatic cancer samples. (2A, 2B) CD24 expression was observed mainly in the cytoplasm of tumour cells. (2C, 2D) CD133 expression was observed mainly in the membrane and cytoplasm of tumour cells, rarely nuclear. (2E, 2F) OCT4 expression was observed in the nucleo of tumour cells, with a focal expression and strong intensity. Lowest magnifying field (40x) are represented in the global panel and highest magnifying field (100x) in the reduced panel.
Regarding surgical treatment, 95.1% of patients undergoing treatment 1 (bileodigestive shunt) were CD133-positive, 70.0% of patients undergoing treatment 2 (partial surgery) were CD133-positive, and 81.2% of patients undergoing treatment 3 (total duodenopancreatectomy) were CD133-positive. There was a significant association (P = 0,019) between surgical treatment and CD133 signal. In addition to surgical treatment, only the sample type was significantly associated with CD133 signal, at a 5% significance level.
The degree of tumor differentiation (good, moderate, or poor) showed no significant association with positive CD133 immunostaining, although it was observed that as the tumor grade progressed, the staining intensity increased (83.7% in poorly differentiated tumors). There was also no significant association between TNM staging and positive CD133 immunostaining, but in later stages the labeling intensifies, even though the presence of CSCs from the earliest stages is observed here. This corroborates the hypothesis that the CSCs have been present since the beginning of the carcinogenesis process.
Table 2 shows the descriptive analysis of the variables together with the verification of their association with CD133 immunoreactivity, given by Fisher’s exact test.
Table 2. Association of variables with positive CD133.
| IHC CD133 positive | p-value* | ||||
| No | Yes | ||||
| N | % | N | % | ||
| Sex | 1,000 | ||||
| Female | 8 | 15.1 | 45 | 84.9 | |
| Male | 9 | 15.5 | 49 | 84.5 | |
| Age | 1,000 | ||||
| 35-59 years | 6 | 16.2 | 31 | 83.8 | |
| 60-69 years | 6 | 15.0 | 34 | 85.0 | |
| 70-93 years | 5 | 14.7 | 29 | 85.3 | |
| Death (n=106) | 0,192 | ||||
| No | 6 | 11.1 | 48 | 88.9 | |
| Yes | 11 | 21.1 | 41 | 78.9 | |
| Surgical Treatment (n=109) | 0,019 | ||||
| Palliative | 2 | 4.9 | 39 | 95.1 | |
| Partial Surgery | 6 | 30.0 | 14 | 70.0 | |
| DPT# | 9 | 18.7 | 39 | 81.3 | |
| Palliative Treatment (n= 91) | 0,474 | ||||
| No | 4 | 18.2 | 18 | 81.8 | |
| Yes | 8 | 11.6 | 61 | 88.4 | |
| Degree of Differentiation (n=107) | 0,084 | ||||
| Well differentiated | 2 | 66.7 | 1 | 33.3 | |
| Moderate differentiated | 9 | 13.4 | 58 | 86.6 | |
| Poor differentiated | 6 | 16.2 | 31 | 83.8 | |
| Specimen/sample (n=107) | 0,028 | ||||
| Metastasis | 7 | 29.2 | 17 | 70.9 | |
| Pancreas | 9 | 10.9 | 74 | 89.2 | |
| Stage T (n=109) | 0,587 | ||||
| T1 | 3 | 25.0 | 9 | 75.0 | |
| T2 | 5 | 12.5 | 35 | 87.5 | |
| T3 | 4 | 19.0 | 17 | 81.0 | |
| T4 | 2 | 8.0 | 23 | 92.0 | |
| TX | 2 | 18.2 | 9 | 81.8 | |
| Stage N (n=109) | 0,830 | ||||
| N0 | 4 | 11.4 | 31 | 88.6 | |
| N1 | 6 | 17.6 | 28 | 82.4 | |
| N2 | 1 | 16.7 | 5 | 83.3 | |
| NX | 5 | 14.7 | 29 | 85.3 | |
| Stage M (n=106) | 0,132 | ||||
| M0 | 8 | 11.3 | 63 | 88.7 | |
| M1 | 9 | 25.7 | 26 | 74.3 | |
| MX | 0 | – | 5 | 100.0 | |
| General stage (n=107) | 0,133 | ||||
| 1 | 2 | 12.5 | 14 | 87.5 | |
| 2 | 5 | 15.6 | 27 | 84.4 | |
| 3 | 1 | 4.0 | 24 | 96.0 | |
| 4 | 9 | 26.5 | 25 | 73.5 | |
| MTX (n=34) | 0,645 | ||||
| Liver | 7 | 30.4 | 16 | 69.6 | |
| Lymph Nodes | 3 | 60.0 | 2 | 40.0 | |
| Peritoneum | 1 | 25.0 | 3 | 75.0 | |
| Others | 1 | 20.0 | 4 | 80.0 | |
| Location: Tumor Head | 0,186 | ||||
| Yes | 12 | 13.2 | 79 | 86.8 | |
| Location: Tumor Tail | 0,071 | ||||
| Yes | 3 | 42.9 | 4 | 57.1 | |
| Location: Tumor Body | 1,000 | ||||
| Yes | 4 | 16.0 | 21 | 84.0 | |
*Fisher’s Exact Test
#DPT: Duodenopancreatectomy
Table 3 show the p-values and associations of variables with intensity when CD133 staining was positive. By Fisher’s exact test (P = 0,036), only stage M showed a significant association with intensity of CD133; that is, tumors that have already metastasized are strongly stained for CD133.
Table 3. P-values of association with intensity.
|
Variables |
Groups |
p-value* | ||
| IHC CD133 –
Intensity when positive |
IHC CD24 –
Intensity when positive |
IHC OCT4 –
Intensity when positive |
||
|
Sex |
Female | 0,222 | 0,438 | 0,619 |
| Male | ||||
|
Death |
No | 1,000 | 0,235 | 0,627 |
| Yes | ||||
|
Surgical Treatment |
Palliative | 0,815 | 0,732 | 0,620 |
| Cir. Partial | ||||
| DPT# | ||||
|
Palliative Treatment |
No | 0,136 | 0,027 | 0,619 |
| Yes | ||||
|
Degree of Differentiation |
Well differentiated | 0,266 | 0,442 | 0,407 |
| Moderate differentiated | ||||
| Poor differentiated | ||||
| Specimen/Sample | Metastasis | 0,848 | 0,201 | 0,488 |
| Pancreas | ||||
|
Stage T |
T1 | 0,611 | 0,992 | 1,000 |
| T2 | ||||
| T3 | ||||
| T4 | ||||
| TX | ||||
|
Stage N |
N0 | 0,560 | 0,920 | 0,180 |
| N1 | ||||
| N2 | ||||
| NX | ||||
|
Stage M |
M0 | 0,036 | 0,350 | 0,078 |
| M1 | ||||
| MX | ||||
|
General Stage |
1 | 0,337 | 0,380 | 0,218 |
| 2 | ||||
| 3 | ||||
| 4 | ||||
|
MTX |
Liver | 0,094 | 0,037 | 1,000 |
| Peritoneum | ||||
| Lymph Nodes | ||||
| Others | ||||
*Fisher’s Exact Test
#DPT: Duodenopancreatectomy
CD24 IMMUNOSTAINING IN CANCER STEM CELLS IN PANCREATIC DUCTAL ADENOCARCINOMA CELLS
CD24 immunostaining (Figures 2A and 2B) was present in the plasma membrane and cytoplasm at both weak/moderate (41.6%) and strong (58.3%) intensities, but at a weaker intensity when compared to CD133 (Figures 2C and 2D). The positive and negative reactions were confirmed with the positive control (placenta) used in the study. Table 4 shows the descriptive analysis of the variables together with the verification of their association with CD24 positivity, given by Fisher’s exact test. A positive CD24 signal was seen in 98.3% of males, but there was no association between sex and CD24 positivity (P = 0,343). Only the type of sample collected and palliative treatment were significantly associated with CD24 signal, at a 5% significance level (P = 0,010 and 0,042 respectively).
Table 4. Association of variables with positive CD24.
| IHC CD24 positive | p-value* | ||||
| No | Yes | ||||
| N | % | N | % | ||
| Sex | 0,343 | ||||
| Female | 3 | 5.7 | 50 | 94.3 | |
| Male | 1 | 1.7 | 58 | 98.3 | |
| Age | 0,159 | ||||
| 35-59 years | 3 | 8.1 | 34 | 91.9 | |
| 60-69 years | 0 | – | 40 | 100.0 | |
| 70-93 years | 1 | 2.9 | 34 | 97.1 | |
| Death (n=107) | 0,618 | ||||
| No | 3 | 5.4 | 52 | 94.6 | |
| Yes | 1 | 1.9 | 51 | 98.1 | |
| Surgical Treatment (n=110) | 0,399 | ||||
| Palliative | 3 | 7.1 | 39 | 92.9 | |
| Partial Surgery | 0 | – | 20 | 100.0 | |
| DPT# | 1 | 2.1 | 47 | 97.9 | |
| Palliative Treatment (n=91) | 0,042 | ||||
| No | 3 | 13.6 | 19 | 86.4 | |
| Yes | 1 | 1.4 | 68 | 98.6 | |
| Degree of Differentiation (n=108) | 1,000 | ||||
| Well differentiated | 0 | – | 3 | 100.0 | |
| Moderate differentiated | 3 | 4.4 | 65 | 95.6 | |
| Poor differentiated | 1 | 2.7 | 36 | 97.3 | |
| Specimen/sample (n=108) | 0,010 | ||||
| Metastasis | 1 | 4.0 | 23 | 95.9 | |
| Pancreas | 2 | 2.4 | 82 | 97.6 | |
| Stage T (n=110) | 0,705 | ||||
| T1 | 0 | – | 12 | 100.0 | |
| T2 | 1 | 2.4 | 40 | 97.6 | |
| T3 | 1 | 4.8 | 20 | 95.2 | |
| T4 | 1 | 4.0 | 24 | 96.0 | |
| TX | 1 | 9.1 | 10 | 90.9 | |
| Stage N (n=110) | 0,881 | ||||
| N0 | 1 | 2.9 | 34 | 97.1 | |
| N1 | 2 | 5.9 | 32 | 94.1 | |
| N2 | 0 | – | 7 | 100.0 | |
| NX | 1 | 2.9 | 33 | 97.1 | |
| Stage M | 1,000 | ||||
| M0 | 3 | 4.2 | 69 | 95.8 | |
| M1 | 1 | 2.9 | 34 | 97.1 | |
| MX | 0 | – | 5 | 100.0 | |
| General Stage (n=108) | 0,913 | ||||
| 1 | 1 | 6.2 | 15 | 93.8 | |
| 2 | 1 | 3.1 | 31 | 96.9 | |
| 3 | 1 | 3.8 | 25 | 96.2 | |
| 4 | 1 | 2.9 | 33 | 97.1 | |
| MTX (n=37) | 1,000 | ||||
| Liver | 1 | 4.3 | 22 | 95.7 | |
| Lymph Nodes | 0 | – | 5 | 100.0 | |
| Peritoneum | 0 | – | 4 | 100.0 | |
| Others | 0 | – | 5 | 100.0 | |
| Location: Tumor Head | 0,550 | ||||
| Yes | 3 | 3.3 | 89 | 96.7 | |
| Location: Tumor Tail | 1,000 | ||||
| Yes | 0 | – | 7 | 100.0 | |
| Location: Tumor Body | 1,000 | ||||
| Yes | 1 | 4.0 | 24 | 96.0 | |
*Fisher’s Exact Test
#DPT: Duodenopancreatectomy
No CD24 immunoreactivity was observed in normal pancreatic ductal epithelium. A positive CD24 signal was seen in 98.6% of patients undergoing palliative treatment, which was significant (P = 0,042). Due to their advanced tumor staging, they had a stronger CD24 signal compared to individuals who underwent total duodenopancreatectomy. The CD24 signal was stronger in individuals who underwent palliative treatment as a treatment option (98.6%) when compared to those who did not (13.6%).
The degree of tumor differentiation and staging showed no significant association with CD24-positive staining, nor were there significant differences between poorly differentiated or advanced-stage tumors compared to well-differentiated and early-stage tumors with respect to positive CD24.
Table 3 show the association of variables with intensity when CD24 was positive. Only the presence of metastasis (MTX) was significantly associated with intensity, where the liver and peritoneum showed a strong positivity when compared to metastasis in other sites, such as lymph nodes and other tissues.
OCT4 IMMUNOSTAINING IN CANCER STEM CELLS IN PANCREATIC DUCTAL ADENOCARCINOMA CELLS
OCT4 immunostaining showed a different pattern in relation to the other antibodies tested. The frequency of positive cases was not as high as observed with the other two antibodies tested, but when positive, its intensity was very strong (Figures 1E and 1F). Immunostaining occurred in the tumor cell nucleus in 31.5% of the cases and did not stain 68.7% of the tumors analyzed. No OCT4 immunoreactivity was observed in normal pancreatic ductal epithelium. No variable was significantly associated with OCT4 signal, at 5% significance level. The mean age of the OCT4-positive group was 62.62 years, and 63.23 years in the OCT4-negative group. However, there was no significant difference between the groups regarding age (P = 0,547).
Although not statistically significant, an interesting finding from the OCT4 staining analysis is that the more differentiated the tumor, the greater the staining intensity, while poorly differentiated tumors had low staining intensity (66.6% vs 21.6%). Cells positive for OCT4 have greater potential for differentiation.
SURVIVAL ANALYSIS
About survival curves and their relation to the three markers, all groups presented very similar curves, so that the log-rank test does not indicate any significant difference between the curves regarding positive signals from the three antibodies. The Kaplan-Meier curves for CD133, CD24, and OCT4 intensity, confirmed by the log-rank test p-values, none of the survival curves differ significantly with respect to intensity. Therefore, the positivity of the markers does not alter the survival of patients included in the study.
DISCUSSION
CSCs have recently been associated with cancer progression and used to identify the prognostic factors and targets for therapy in several types of cancer (DEAN et al., 2005). It is notable that the role of CSCs is not well understood or characterized in pancreatic cancer. Moreover, few reports have been published on the patterns and effects of the presence of CSCs. Considering this lack of data, we retrospectively analyzed the expression of CSCs in samples of pancreatic cancer using immunohistochemistry and verified its role in tumor progression and therapeutic resistance in ductal pancreatic adenocarcinoma.
The molecular biology of pancreatic cancer is directly related to its rapid progression and low therapeutic response. It is well documented that the appearance of a tumor cell is the result of an accumulation of mutations in its DNA capable of morphologically and functionally altering it (FANG et al., 2013; RICCI-VITIANI et al., 2007). The trigger of this process and all its evolution, culminating in the formation of the tumor cell and its pertinent characteristics, characterize the carcinogenesis process, is already significantly elucidated for several types of neoplasias. However, for certain types of cancer such as pancreatic cancer, the process remains uncertain (KOBAYASHI; NORONHA, 2015).
Most malignant tumors follow a natural history that can be divided into four stages: malignant transformation, tumor cell growth, local invasion, and metastasis. The process of carcinogenesis, or malignant transformation, occurs in various stages and results due to the accumulation of genetic changes and mutations, which are triggered by several factors, including environmental factors such as radiation and chemicals (AYOB; RAMASAMY, 2018). However, for this process of carcinogenesis to progress, it may be necessary to have certain cells with the stem cell characteristics, such as the potential for self-renewal and regeneration, as well as a high proliferative index.
Recent evidence suggests that stem cells play a crucial role in not only the generation and maintenance of different tissues but also the development and progression of certain types of tumors (BALIC et al., 2012). For several solid tumors, they have been shown to harbor a distinct subpopulation of tumor cells that have stem cell characteristics and are therefore called tumor stem cells (CTTs) or tumorigenic cells (MATSUDA et al., 2012). Cancer stem cells appear to be able to initiate and drive tumour growth in solid tumours. CD133, CD24 and OCT4 are recently reported prospective markers for CSCs, expressed in a variety of tumours; however, to our knowledge, no attempt has been made to detect their expression in pancreatic cancer specimens (MAEDA et al., 2008).
It can be observed that tumor stem cells, or tumor stem cell surface markers such as CD133, CD24, and OCT4 are present in pancreatic cancer tumor cells, confirming the intrinsic relationship between the pancreatic malignant phenotype and the presence of stem cells. No significant difference was observed between antibody expression and the degree of tumor differentiation, assuming that tumor stem cells have been present since tumor initiation. It should be noted that for CD133 and CD24 antibodies, the percentage of marker-expressing tumor cells increased as the tumor progressed. Hence, the more aggressive, poorly differentiated tumors exhibited greater positivity for the presence of the antibody. While on the other hand, the expression of the OCT4 antibody was higher and more intense in well-differentiated tumors; thus, the more differentiated the tumor, the greater the labeling. Cells that have the stem cell marker for the OCT4 antibody have a higher potential for differentiation (VILLODRE et al., 2016). This may be the reason why OCT4 differed in terms of expression from the other markers that were tested, although these differences were not statistically significant.
OCT4 marker is only present focally in tumor samples that were positive. This find is in agreement with the theory that CSCs represent only a very small portion of the total tumour cell population (PHI et al., 2018). For example, CD133 positive cells were detected in 0.7-6.1% (RICCI-VITIANI et al., 2007), 1.8-24.5% (O’BRIEN et al., 2007) of primary colon cancer cells, and in 0.7-3.2% of primary pancreatic cancer cells using flow cytometric analysis (HERMANN et al., 2007). Immunohistochemical staining revealed CD133 expression in 1-3% of hepatocellular carcinoma specimens (MA et al., 2007).
Although labeling and expression were observed with a relatively high frequency in all degrees of tumor direction, for example, tumors expressed labeling for cancer stem cells regardless of the degree of differentiation, some findings favor the hypothesis that the number of stem cells in tumors is larger in tumors with more aggressive behavior and with worse prognosis. This hypothesis is supported by the fact that CD133 was more frequently expressed in individuals who underwent only palliative treatment (95.1%) with a significant association (p-value < 0.05) between the presence of antibody and palliative treatment. When it is reported that a patient underwent only palliative treatment, it is assumed that the tumor was at a significantly advanced stage and with no possibility of curative surgical intervention and therefore only palliative treatment was performed. Similarly, it can be overstated that the more aggressive tumors at later stages express greater labeling for the CD133 tumor stem cell antibody. Several clinical studies have investigated the relationship between the CD133 expression and clinical outcomes of tumors; however, the results are controversial. For example, CD133 expression is related to the poor prognosis of colon cancer and hepatocarcinoma (CAI et al., 2018).
Regarding the TNM stage, the labeling of the three antibodies (CD133, CD24, and OCT4) did not change along the progression. It is notable that the tumor stem cells were present from the earliest stage and that they do not enlarge during the staging. The prevalence of the three antibodies and their associations with the variables was generally high, although not all the antibodies had a significance level lower than P < 0.05.
All the variables that contributed to an unfavorable prognosis, such as the location of the pancreatic head tumor, advanced age, and the presence of metastases were all associated with high prevalence especially for the expression of CD133 and CD24. Additionally, this corroborated with the idea that tumor stem cells when present are associated with rapid tumor progression and therapeutic resistance. Cancer stem cells have certain characteristics similar to those of normal organism stem cells, such as the ability to self-regenerate and pluripotency, giving rise to heterogeneous tumor cell lines that form the same tumor (TAI et al., 2005). Cancer stem cells are likely to share many of the properties of normal stem cells that provide for a long lifespan, including relative quiescence, resistance to drugs and toxins through the expression of several transporters, an active DNA-repair capacity and a resistance to apoptosis. Therefore, tumours might have a built-in population of drug-resistant pluripotent cells that can survive chemotherapy and repopulate the tumour (MARQUES et al., 2010).
In order to define if CSCs predicted the clinical outcome, we performed Kaplan-Meier survival analysis and showed that the groups presented very similar curves. Hence, the log-rank test does not indicate significant difference between the curves regarding the presence of the three antibodies. Although they are associated with worse prognostic variables such as metastasis and palliative treatment therapeutic options, no association can be made between the presence of tumor stem cells and patient survival in this case; therefore, the presence of CSCs does not predict worse survival.
The discovery of cancer stem cells in solid tumours has changed our view of carcinogenesis. That tumor stem cells are involved in tumor development and progression is a fact, since their presence were significant in the vast majority of tissues analyzed and, recently, many studies have associated the presence of tumor stem cells with the development of several types of cancer.
FINAL CONSIDERATIONS
The process of tumorigenesis, as far as the subject is known until now, involves the progressive acquisition of multiple aberrant mutations that culminate in the expansion and proliferation of more aggressive cell subpopulations, contributing to tumor progression and growth. Although this theory is well established, the identification of cells that are capable of causing the tumor progression is still the subject of many studies to better characterize the carcinogenesis process and identify these cells.
Much of the information we obtain and use for the diagnosis, prognosis, and treatment of pancreatic neoplasms derives from heterogeneous populations with varying degrees of maturation and differentiation. It is becoming increasingly urgent to characterize the CSCs for the evaluation and characterization of the carcinogenic route of the most aggressive tumors. The direct consequence is the development of therapeutic strategies that can act on CSCs and the possibility to determine, by an easily accessible method, which patients will benefit most from this type of treatment.
REFERENCES
ALLEGRA, E.; TRAPASSO, S. Cancer stem cells in head and neck cancer. Onco Targets Ther., v.5, p.375-83, 2012.
ANSARI, D.; CHEN, B.C.; DONG, L.; ZHOU, M.T.; ANDERSSON, R. Pancreatic cancer: translational research aspects and clinical implications. World J Gastroenterol., v.18, n.13, p.1417-24, 2012.
AYOB, A.Z.; RAMASAMY, T.S. Cancer stem cells as key drivers of tumour progression. J Biomed Sci., v.25, n.20, 2018.
BALIC, A.; DORADO, J.; ALONSO-GÓMEZ, M.; HEESCHEN, C. Stem cells as the root of pancreatic ductal adenocarcinoma. Exp Cell Res., v.318, n.6, p.691-704, 2012.
BARBOSA, I.R.; SANTOS, C.A.; SOUZA, D.L.B. Pancreatic cancer in Brazil: mortality trends and projections until 2029. Arq. Gastroenterol., v.55, n.3, p.230-6, 2018.
CAI, X.; LI, J.; YUAN, X.; XIAO, J.; DOOLEY, S.; WAN, X., WHENG, W.; LU, L. CD133 expression in cancer cells predicts poor prognosis of non-mucin producing intrahepatic cholangiocarcinoma. J Transl Med., v.16, n.50, 2018.
DEAN, M.; FOJO, T.; BATES, S. Tumour stem cells and drug resistance. Nat Rev Cancer., v.5, n.4, p.275-84, 2005.
FANG, Y.; YAO, Q.; CHEN, Z.; XIANG, J.; WILLIAM, F.E.; GIBBS, R.A.; CHEN, C. Genetic and molecular alterations in pancreatic cancer: implications for personalized medicine. Med Sci Monit., v.19, p.916-26, 2013.
HERMANN, P.C.; HUBER, S.L.; HERRLER, T.; AICHER, A.; ELLWART, J.W.; GUBA, M.; BRUNS, C.J.; HEESCHEN, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell., v.1, n.3, p.313-23, 2007.
HU, Y.; FU, L. Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res., v.2, n.3, p.340-56, 2012.
KOBAYASHI, N.C.C.; NORONHA, S.M.R. Cancer stem cells: a new approach to tumor development. Rev Assoc Med Bras., v.61, n.1, p.86-93, 2015.
KONONEN, J.; BUBENDORF, L.; KALLIONIMENI, A.; BÄRLUND, M.; SCHRAML, P.; LEIGHTON, S.; TORHORST, J.; MIHATSCH, M.J.; SAUTER, G.; KALLIONIMENI, O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med., v.4, n.7, p.844-7, 1998.
LI, D.; JIAO, L. Molecular epidemiology of pancreatic cancer. Int J Gastrointest Cancer., v.33, n.1, p.3-14, 2003.
MA, S.; CHAN, K.W.; HU, L.; LEE, T.K.W.; WO, J.Y.H.; NG, I.O.L.; ZHENG, B.J.; GUAN, X.Y. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology, v.132, n.7, p.2542-56, 2007.
MAEDA, S.; SHINCHI, H.; KURAHARA, H.; MATAKI, Y.; MAEMURA, K.; SATO, M.; NATSUGOE, S.; AIKOU, T.; TAKAO, S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer., v.98, n.8, p.1389-97, 2008.
MARQUES, D.S.; SANDRINI, J.Z.; BOYLE, R.T.; MARINS, L.F.; TRINDADE, G.S. Relationships between multidrug resistance (MDR) and stem cell markers in human chronic myeloid leukemia cell lines. Leuk Res., v.34, n.6, p.757-62, 2010.
MATSUDA, Y.; KURE, S.; ISHIWATA, T. Nestin and other putative cancer steam cell markers in pancreatic cancer. Med Mol Morphol., v.45, n.2, p.59-65, 2012.
O’BRIEN, C.A.; POLLETT, A.; GALLINGER, S.; DICK, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, v.445, n.7123, p.106-10, 2007.
PHI, L.T.H.; SARI, I.N.; YANG, Y.G.; LEE, S.H.; JUN, N.; KIM, K.S.; LEE, Y.K.; KWON, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem Cells Int., n.5416923, 2018.
RICCI-VITIANI, L.; LOMBARDI, D.G.; PILOZZI, E.; BIFFONI, M.; TODARO, M.; PESCHLE, C.; DE MARIA, R. Identification and expansion of human colon-cancer-initiating cells. Nature, v.445, p.111-5, 2007.
SAKORAFAS, G.H.; TSIOTOU, A.G.; TSIOTOS, G.G. Molecular biology of pancreatic cancer; oncogenes, tumor suppressor genes, growth factors, and their receptors from a clinical perspective. Cancer Treat Rev., v.26, n.1, p.29-52, 2000.
SOTO, J.L.; BARBERA, V.M.; SACEDA, M.; CARRATO, A. Molecular biology of exocrine pancreatic cancer. Clin Transl Oncol., v.8, p.306-12, 2006.
TAI, M.H.; CHANG, C.C.; OLSON, L.K.; TROSKO, J.E. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis, v.26, n.2, p.495-502, 2005.
VILLODRE, E.S.; KIPPER, F.C.; PEREIRA, M.B.; LENZ, G. Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev., v.51, p.1-9, 2016.
[1] MSc in Medical Sciences, Postgraduate Program in Medicine: Medical Sciences, Federal University of Rio Grande do Sul (UFRGS), Brazil.
[2] PhD in Physiological Sciences, Federal University of Rio Grande (FURG), Brazil.
[3] MSc in Genetics and Molecular Biology, Federal University of Rio Grande do Sul (UFRGS), Brazil.
[4] Undergraduate Medical Student in Federal University of Rio Grande (FURG), Brazil.
[5] PhD in Biological Sciences (Biochemistry) in Federal University of Rio Grande do Sul (UFRGS), with sandwich doctorate in neuroscience – University of California Irvine.
Submitted: September, 2020.
Approved: October, 2020.