ARTIGO DE REVISÃO
REIS, Ariadna Jihani Damasceno Vidal de Santana [1], ANJOS, Maria Clara Mota Nobre dos [2], CAVALCANTI, Bruna Luiza de Mendonça [3], SANTOS, Ketully Stefane Chaves dos [4], DINIZ, Maria Celeste Campello [5]
REIS, Ariadna Jihani Damasceno Vidal de Santana. Et al. Posttraumatic stress disorder as a trigger of epigenetic changes in the body. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 08, Vol. 04, pp. 96-114. August 2020. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/epipgenetic-changes
SUMMARY
Posttraumatic stress disorder (PTSD) has as diagnostic definition the occurrence of severe traumatic stress as an etiological triggering factor, and after exposure to this traumatic event begins a variable series of characteristic symptoms and psychological, social and biological disorders. This article systematically evaluates how PTSD can influence the hypothalamus-pituitary-adrenal axis by epigenetic modifications that suppress or stimulate the genetic expression of a given gene through biomarkers. Research of qualitative approach, with nature of the type systematic bibliographic review of analytical character. Studies were researched that sought to correlate PTSD with epigenetic alterations in subsequent generations. The research was constructed through queries to NCBI databases: PubMed, BVS and Scielo. The information was collected from 14 scientific articles published between 2009 and 2018, and the languages were delimited to Portuguese and English. It was observed that PTSD is responsible for a considerable change in cortisol, serotonin and sensitivity to glucocorticoide receptors. The review allowed the construction of a synthesis of scientific knowledge about the association between epigenetic changes motivated by PTSD in patients in genetic material and such modifications of these genes will be transferred to future generations.
Keywords: DNA methylation, epigenetics, biomarkers, posttraumatic stress disorders.
1. INTRODUCTION
Posttraumatic stress disorder (PTSD) is defined as a set of psychic, physical and emotional signs and symptoms that cause an anxiety disorder. PTSD is responsible for a considerable change in cortisol, serotonin and sensitivity to glucocorticoid receptors (GRS). Thus, it develops in the individual who was a victim or witness to a traumatic situation that presented threat to his integrity or that of people around him. This disorder causes hormonal changes linked to the hypothalamic-pituitary-adrenal (HPA) axis and in the expression of certain gene (VUKOJEVIC et al., 2014). In the meantime, the relationship between stress, PTSD, and epigenetics becomes clear, because even if it does not involve change itself in the sequence of the genetic code, there are alterations that can effect the stimulation or inhibition of gene expression of certain information. Such changes are the methylation of deoxyribonucleic acid (DNA) and changes in histonas that present the role of normal development and are crucial for the performance of the correct programming of gene expression.
In this article, we will discuss mainly the process of DNA methylation of the main genes of GRS, mineralocorticoids (MR) and serotonin (HTR3A) that promotes an adaptation to the body and influence on susceptibility to diseases that would consequently also be propagated the next generations.
The HTR3A, NR3C1, NR3C2 and FKBP5 genes will focus on this article. First, hypermethylation of the NR3C1 gene results in its low expression and decreased cortisol levels. NR3C2 is responsible for the drop in MR levels and, consequently, for the decrease in the amount of corticotropin. This process occurs through methylation of the gene that expresses NR3C2 (PERROUD, 2014). HTR3A may be associated with modification in brain structures central to the processing of emotions, especially when these structures are exposed to stress. It is also noteworthy the potential influence of the serotonergic system on the pathophysiology of affective disorders, such as PTSD. Finally, FKBP5, which acts as a co-chaperone, modulating the activity of RG in response to stressors during pregnancy and is also related to exposure to trauma generates the possibility of the child already being born with a mechanism more adapted and prone to develop depression and PTSD (PAQUETTE et al., 2014); (SCHECHTER et al., 2016).
1.1 EPIGENETICS
The term epigenetics means “in addition to genetic information encoded in DNA” and is generically used to define changes that occur in gene expression without, however, any alteration in the sequence of the genetic code (COSTA et al., 2013).
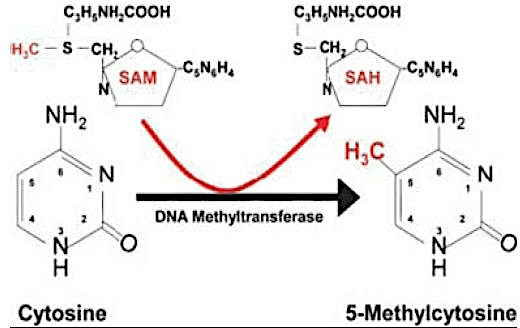
Some epigenetic mechanisms include DNA methylation, imprinting, changes in chromatin conformation, and RNA-mediated silencing. DNA methylation is the epigenetic modification most commonly used by most living beings as a gene expression regulation agent, and can be characterized as a gene silencing factor. It consists of the addition of a methyl radical (CH3) to carbon 5 of Cytosine, usually followed by Guanine (dinucleotide CpG), catalyzed by enzymes DNA methyltransferases (DNMTs) (COSTA et al., 2013); (AMLI et al., 2016).
Figure 01. Cytokine methylation.
1.2 HPA SHAFT ADJUSTMENT
Hpa axis activity is governed by the secretion of HLC – corticotrophic and vasopressin-releasing hormone (AVP) – by the hypothalamus, which in turn activates the secretion of the adrenocorticotrophic hormone (ACTH) by the pituitary gland, which ultimately stimulates the secretion of glucocorticoids through the adrenal cortex. In this view, glucocorticoids interact with their receptors in multiple target tissues, including the HPA axis itself, where they are responsible for inhibiting negative feedback of ACTH secretion by the pituitary and HLC from the hypothalamus (JURUENA et al., 2004).
Although glucocorticoids adjust the functions of almost all tissues in the body, the best known physiological effect of these hormones is the regulation of energy metabolism. In this context, several factors control hpa axis activity. In addition, there is evidence of direct catecholaminergic, serotoninergic and dopaminergic innervation in HLC-producing neurons in the hypothalamus and these and other neurotransmitters seem to influence the release of HLC. For example, serotonin exerts a stimulating influence on HLC through receptor subtypes 5-HT1A, 5-HT1B, 5-HT1C and 5-HT2. Norepinephrine has a more variable effect, being stimulator in low doses (via alpha 1 receptors) and inhibition in high doses (via beta receptors) (JURUENA et al., 2004).
This article aimed to discover the epigenetic relationship of gene expression in PTSD through the aforementioned biomarkers. From the study of these, their interaction with the regulation and performance in the hypothalamus-pituitary-adrenal axis was investigated. Finally, we sought to understand how such changes will act in the transfer of these genes to future generations.
2. METHODOLOGY
The research was developed with a qualitative approach, which is justified by allowing the unsealing of the researched world with deepening of scientific data, which allows a better understanding and analysis of the research object. The nature of the study consisted of a systematic literature review of an analytical nature. Justifying itself for assisting in the construction of a broad analysis of the literature, contributing as a reflection to the realization of future studies. The bias was delimited with the descriptors stress and epigenetics. A total of 77,937 articles were used for consultation in the Virtual Library, such as Lilacs, virtual health library (BVS), Scielo and MEDLINE via Pubmed, a total of 77,937 articles that were submitted to a new review conducted by reading the titles that also allowed an inclusion criterion that would be the article dealing with PTSD; this preliminary screening excluded 76,359 articles.
By reading the title under the criterion of being related to PTSD. These filters reduced the number of articles from about 80,000 to 1,578 articles. This systematization of how the research was performed is defined in figure 02.
The new stage of the thematic specification included the inclusion of new inclusion and exclusion criteria, such as: human race, women, publications up to 5 years old, exclusion of articles review, aged between 19-44 years. The 89 articles resulting from this filtering, went through an analysis in which there was the removal of duplicate articles allied to the exclusion and inclusion criteria, decreasing the number of articles to 14 articles that were submitted to another analysis that involved the reading of these articles so that the group could select what would be more relevant for a review.
There was a focus on articles on victims of disasters, genocides or large-scale traumatic events that, therefore, culminated in the exclusion of articles that addressed the theme, but did not correspond to our objectives for the writing of this review article, only eight articles useful for the thematic section chosen by the group. The articles chosen were organized and represented in Table 01.
Figure 02. Flowchart demonstrating the way the exclusion line of the systematic review was performed
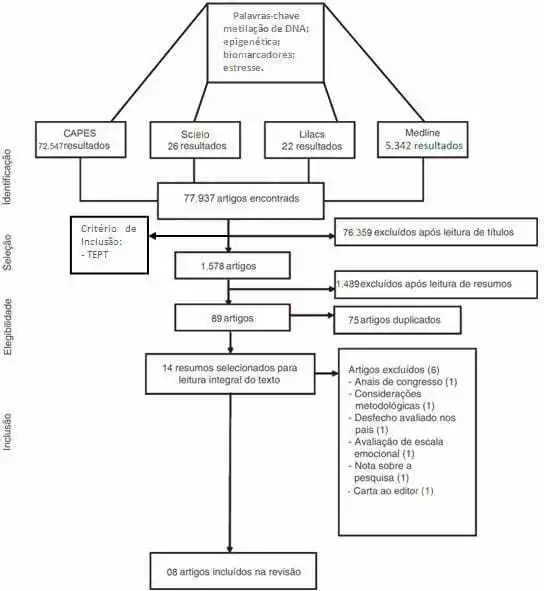
Table 01 List of authors and their respective articles and presentation of the results found.
| Author | Articles | Study | Goals | Results |
| McNerney, MW et al (2018) 8 | Neural and epigenetic integration contributions to the symptoms of post traumatic stress disorder : The role of hippocampus volume and methylation of the glucocorticoides gene. | Research Article | The article seeks to determine whether the combination of brain and epigenetic images is a better predictor of PTSD symptomatology than any factor alone.Measuring hippocampus volume and cytosine methylation. | The data revealed a significant interaction between NR3C1 methylation and hippocampus size. The findings strengthen the idea that epigenetics and neural anatomy can be used as an effective indicator for PTSD |
| Schechter D.S et al (2016) | Association of serotonin receptor 3A methylation with exposure to maternal violence, neural activity and child aggression. | Research Report | The study in question examined whether HTR3A methylation may be associated with the mother’s exposure to interpersonal violence (IPV), IPV-related psychopathology, childhood attachment disorder, and maternal neural activity. |
The frequency of exposure to maternal IPV was associated with maternal PTSD; and maternal PTSD-PTSD, in turn, was associated with child SOD. |
| Perroud N et al(2014) | The Tutsi genocide and the transgenerational transmission of maternal effort: epigenetics and biology of the HPA axis. | Research Article | The study of the transmission of parental posttraumatic stress disorder (PTSD) to offspring that can be explained by the transmission of epigenetic processes, such as the methylation status of the NR3C1 gene and the glucocorticoid receptor (GR) | There was a significant correlation between the severity of PTSD and depression in mothers and the severity of PTSD and depression in their children, respectively. |
| Paquette A.G et al (2014) | Posttraumatic stress disorder in patients with bipolar disorder: a review of prevalence, correreports and treatment strategies. | Cohort Study | The study aims to test the hypothesis that the methylation of placental FKBP5 and genetic variation contribute to the control of gene expression, and are associated with the outcomes of child neurodevelopment. | Data show that infants with the highest levels of methylation would experience a reduction in FKBP5 expression, resulting in increased cortisol activation in glucocorticoid receptors within the placenta, which may influence the activation of the glucocorticoid response pathway in the developing baby. |
| Lynn M. et al (2016) | A genome-wide risk variant for PTSD is a locus of quantitative methylation characteristic and confers decreased cortical activation and fearful faces | Cohort Study | This study aims to understand the genetic risk for PTSD. A sample with an extreme phenotype design and control cases with similar exposures was observed. | The BSBPCohort had a general mean of PTSD symptom scores. However, as the cohort was developed with extreme phenotypes, the severity of symptoms was much higher in cases of PTSD. |
| Vukojevic et al (2014) | Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and the risk of posttraumatic stress stress in genocide survivors. | Behavioral/Cognitive | It aims to analyze the traumatic events that induce DNA methylation, and which provides changes in different promoters of RG and transcriptional modifications, in addition to an analysis of the regulation of cortisol activity. Thus associating these factors with the hypothalamus-pituitary-hypophysis-hypophysis-adrenal axis in individuals with PTSD. | The analysis also revealed a main effect of cortisol sampling time. Partial tests indicated that cortisol levels were significantly lower in the PTSD group compared to the control group for the time interval between 30 and 45 minutes after awakening (and between 45 and 60 minutes after awakening, glucocorticoid receptors were significantly lower in PTSD compared to the other comparison group.) |
| Kaminsky Z et al ( 2015) | Epigenetic and genetic variation in suicidal behavior predicate of ska2 and posttraumatic stress disorder. | Original Article | The objective was to investigate the interaction of SKA2 and exposure to trauma in hpa axis function, suicide attempt and PTSD | It was observed that the epigenetic and genetic biomarker, SKA2, predicted cases of PTSD in civilians when child abuse was incorporated |
| MULLIGAN C.J. et al (2012) | Methylation in NR3C1 in newborns associated with exposure to maternal prenatal stress and newborn weight | Integrated Article | The study aims to test the idea that extreme maternal psychosocial stressors as observed in the Democratic Republic of congo can modify epigenetic marks of specific locus in the newborn, resulting in altered health outcomes. | It concluded that increased methylation may restrict the malleability of gene expression and restrict the range of possible stress adaptation responses in affected individuals, thus increasing the risk of diseases with PTSD at adulthood. |
Source: Authors of the research (2019).
3. RESULTS AND DISCUSSION
3.1 HYPERMETHYLATION, HYPOMETHYLATION AND PTSD
The methylation process plays key roles in embryonic development, X chromosome inactivation, gene regulation, genomic imprinting and chromatin modifications. However, there are two mechanisms for regulating gene expression within the methylation process: hypermethylation and hypomethylation. Dna hypomethylation commonly causes an increase in gene expression, but hypermethylation decreases the expression of determined genes (VUKOJEVIC, 2014).
Naturally, the methylation patterns of the genome change with the arrival of senescence. Most of the time, hypomethylation occurs concomitantly with hypermethylation of some CPG islands distributed in recurrent sequences, as well as transcriptionally important genes. Epigenetic changes are directly related to advancing age, contributing to various acquired disorders or diseases (FRAGA et al., 2005).
Repeated exposure to stress during childhood predisposes a neurobiological reaction that is maintained throughout the individual’s life, and can negatively affect psychic health. Previous studies have effectively shown that environmental stressors, not only early in life, but also during pregnancy, increase the methylation state of the 1F exon of the NR3C1 gene. In the brain, the hippocampus is an abundant area in GRS, which also expresses the promoter NR3C1-1F. In addition, there are records that the hippocampus is sensitive to stress at a biochemical and structural level. Therefore, DNA methylation has been associated with changes in RG density in the hippocampus after exposure to environmental stress. Therefore, studies indicate that, either due to exposure to stress and possible loss of hippocampus volume or by the state of ininate hypomethylation combined with a lower hippocampus volume, they are able to predispose an individual to PTSD (MCNERNEY et al., 2018).
It cannot be affirmed that PTSD is associated with hippocampus volume. Although this controversy can be justified by the reason that not all important variables involved in hippocampus volumes and PTSD were completely considered as the DNA methylation mechanism (PERROUD et al., 2014).
The performance of hypermethylation is able to effectively disturb the balance of the HPA axis, which explains how cortisol levels (YEHUDA et al., 2009) altered are found in the children of disaster survivors diagnosed with PTSD. Thus, epigenetics can provide important information about the pathophysiology of PTSD, making it possible to understand how transgenerational transmission of this disorder occurs (PERROUD et al., 2014); (JURUENA et al., 2004).
Epigenetic alteration in ptsd carrier and propagation for their offspring.
This epigenetic alteration is directly related to the HPA axis, which is basically responsible for the neuroendocrine regulation of physiological processes. However, for a person with PTSD, the functioning of this axis changes as a form of adaptation that can be related to low cortisol levels. Therefore, several studies suggest that this process is linked to the beginning of the development of psychic diseases, and probably has its appearance from the uterus, as a consequence of the reflection of glucocorticoid programming through epigenetics (BRAND et al., 2006).
These studies indicate that the increase in methylation of the NR3C1 gene, responsible for rg coding, causes mr imbalance – encoded by the NR3C2 gene – essentially resulting in the reduction of the availability of RG providing greater susceptibility of this individual to stress-related psychiatric diseases, such as depression and PTSD (BRAND et al., 2006; MULLIGAN et al., 2012)
Added to this factor of alteration of the structure in the DNA of people who suffer interference from external stressors, several studies suggest that PTSD is a strong correlated for the development of this same pathology in future generations (ROBERTS et al., 2012). Transgenerational propagation is associated with the transmission of adaptive biological changes on the HPA axis. PTSD causes the change in the neuroendocrine balance of the HPA axis, especially when it comes to the secretion of low cortisol levels and this low secretion is also increased by anti-inflammatory drugs that are part of the group of corticosteroids that instill cortisol production by the body, also enhancing its low secretion, which consequently increases the probability of the offspring of parents with PTSD acquiring the disease in the future (BRAND et al. , 2006), (LABONTÉ et al., 2014). The low cortisol level in relation to parental PTSD seems to be present in the early development of the baby – probably from the uterus- since they cause the reprogramming of GRS through epigenetic alterations that promote the appearance of disorders in genetic expression (RADTKE et al., 2011). Fetal response to cortisol level indicates that exposure to maternal gestational stress has lasting impacts on the development of this child through increased methylation of the RG-promoting gene that persists beyond childhood (RADTKE et al., 2011), (LABONTÉ et al., 2014). This represents fetal adaptive programming of the HPA axis, which may be responsible for later psychological problems such as depression or PTSD. From this perspective, the fetus exposed to altered cortisol levels in the maternal circulation is associated with neurological and developmental problems of the next generation (BRAND et al., 2006).
The placenta, the central organ of fetal development, regulates exposure and feedback to maternal signaling, altering the fetal development environment. Epigenetic mechanisms, such as DNA methylation, when they occur in regulatory regions, alter placental function, modifying gene transcription or transcription potential (PAQUETTE et al., 2014). FKBP5 (Binding Protein FK506) decreases RG sensitivity and prevents nuclear translocation, which consequently reduces cortisol release responsiveness (KAMINSKY et al., 2015). Methylation of FKBP5 within the regulatory region at intron 7 is associated with neurological changes. It was observed that intron 7 binds to the site of beginning of FKBP5 transcription and methylation alters the induction of FKBP5. In a human hippocampus cell line, cortisol induces the demethylation of this regulatory region within intron 7 during critical periods of cell differentiation and proliferation. FKBP5 polymorphism is also linked to altered mrna induction, which determines changes in hippocampus size, as well as diseases related to changes in the HPA axis such as PTSD (SCHECHTER et al., 2016); (KAMINSKY et al., 2015).
Studies show that the fetus with the gene located in hypermethylated intron 7 would experience a reduction in FKBP5 expression, resulting in a greater sensitivity of GRS to cortisol from the maternal circulation within the placenta, thus influencing fetal development. Studies prior to the FKBP5 biomarker have observed associations with PTSD that are characterized by hypervigilant and hyperexcited behavior. Therefore, changes in FKBP5 decrease cortisol response influence behavior by promoting a hypervigilant response. Individuals with increased methylation of FKBP5 in utero have a little-active cortisol response pathway at birth, becoming predisposed to develop disorders related to hpa axis hyperresponsiveness, such as PTSD or anxiety (KAMINSKY et al., 2015). Next, it was analyzed that this biomarker has its functional relevance for an epigenetic control still unknown and can alter fetal development, especially in neurobehavioral development. Thus, studies effectively demonstrate that external stressors, actively affecting during pregnancy, cause hypermethylation of the 1F ethyl of the NR3C1 gene and the region of the RG promoter in order to significantly interfere in adulthood (RADTKE et al., 2011).
This phenomenon is relevant for prolonged modifying the balance of the HPA axis and altering cortisol levels found in the children of victims of natural or anthropogenic disasters who have been diagnosed with PTSD. Thus, epigenetics can provide information on the behavior of biomarkers and macrostructural manifestations in order to clarify the transgenerational transmission of this disorder.
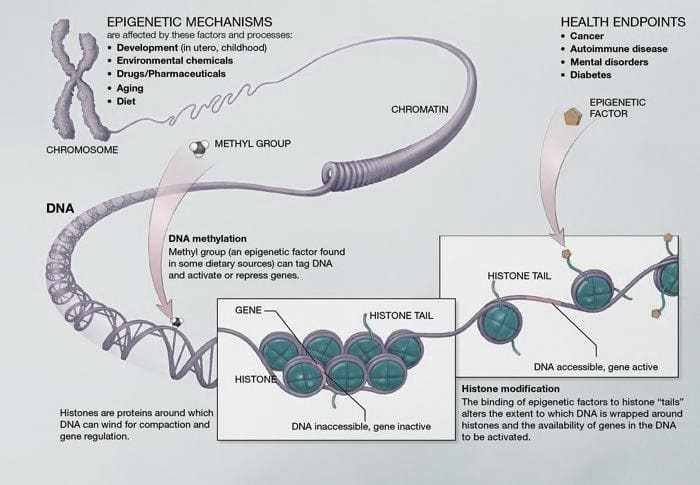
Figure 03. DNA methylation- Base pairs form DNA. These pairs intertwine in proteins called histones. This structure is similar to a “pearl necklace” that forms chromatin. Methylation is the main epigenetic mechanism.
3.2 THE ROLE OF EPIGENETIC CHANGE IN HIPPOCAMPUS VOLUME AND GLUCOCORTICOID RECEPTOR
Currently, methylation of the NR3C1 gene is correlated with hippocampus volume and depression. The hippocampus is sensitive to stress and highly responsive to methylation of this biomarker. Relating these factors to neuroimaging and epigenetic mechanisms, they were used to provide the basis for an interesting orientation in research related to PTSD and genotype changes. In general, PTSD revealed a negative response between NR3C1 and hippocampus size. In addition, a strong relationship between this biomarker, hippocampus, GRS and ptsd response. Specifically, the hippocampus regulates hpa axis activity and consequently the action of glucocorticoids, such as cortisol. Thus, increasing the signs of stress in the body significantly (PERROUD et al., 2014), (AMLI et al., 2016).
This would lead us to expect that lower methylation values are associated with a smaller-sized hippocampus, which war veterans who probably don’t have PTSD should have. However, PTSD is associated with hypomethylation in general and it is verified that this relationship is lost because lower methylation values can also occur, regardless of hippocampus size. Future research should be conducted to determine whether this trend remains the same in healthy individuals, other veterans or civilian groups with PTSD, and in patients with other psychiatric disorders (AMLI et al., 2016).
The relationship between hippocampus volume and PTSD is currently controversial in the literature. Imaging studies indicate that post-combat PTSD veterans have a smaller hippocampus volume compared to veterans without PTSD. However, other studies have failed to replicate this relationship. The probable reason for this inconsistency in the results is due to the fact that PTSD is a multifactorial disorder (AMLI et al., 2016), (LABONTÉ et al., 2014). Therefore, understanding the biological basis of this disorder requires more than just neuroimaging to analyze the relationship of PTSD with the volume and functioning of the hippocampus. Therefore, it is imperative to integrate the knowledge of imaging, biochemistry and epigenetics in order to deepen the current knowledge about this disorder.
4. FINAL CONSIDERATIONS
In this article, after studying the biomarkers cited that are expressed or not by epigenetic changes, we can point out some of the most current results in research on PTSD and follow how these articles provide interesting and varied perspectives for the premature discovery of predisposition to certain psychological disorders. Although the articles are not conclusive, the results of their studies indicate that traumatic events in PTSD in the body macrostructure are justified through DNA methylation alterations that modify gene expression and, consequently, HPA axis activity. Therefore, PTSD not only produces changes in the neuroendocrine control axis of the body, but also modifications can be propagated to future generations that alter the mechanism of offspring to act and similarly with the mechanism of its parent.
Although the range of information on the subject addressed was broad, some articles studied revealed the need for further research to obtain a deeper study on the subject. Thus, it would be possible to consolidate the information obtained in the aforementioned studies.
REFERENCES
AMLI, Lynn M. et al. A Genome-Wide Identified Risk Variant for PTSD is a Methylation Quantitative Trait Locus and Confers Decreased Cortical Activation to Fearful Faces. Am J Med Genet B Neuropsychiatr Genet. Georgia, p. 327-336. 18 maio 2018. Disponível em: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844461/>. Acesso em: 22/11/ 2019.
BRAND, Sarah R. et al. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Annals of the New York Academy of Science. Nova Iorque, p. 454-458. abr. 2006. Disponível em: <https://nyaspubs.onlinelibrary.wiley.com/doi/full/10.1196/annals.1364.041>. Acesso em: 30/10/2019.
COSTA, Everton de Brito Oliveira; PACHECO, Cristiane. Epigenetics: gene expression regulation at transcriptional level and its implications. Semina. Londrina, p. 125-136. dez. 2013. Disponível em: <http://www.uel.br/revistas/uel/index.php/seminabio/article/viewFile/5142/13877>. Acesso em: 01 fev. 2019
FRAGA, Mario F. et al. Epigenetic differences arise during the lifetime of monozygotic twins. Epigenetics Differences Arise During The Lifetime Of Monozygotic Twins. Proceedings Of The Proceedings Of The National Academy Of Sciences Of The United States Of America. Seattle, p. 106-117. 17 jan. 2005. Disponível em: <https://www.pnas.org/content/102/30/10604.long>. Acesso em: 27/10/2019.
HARRIS, Angela P. et al. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology. Edimburgo, p. 648-658. maio 2013. Disponível em: <https://www.sciencedirect.com/science/article/pii/S0306453012002983?via%3Dihub>. Acesso em: 11/09/2019
https://www.tandfonline.com/doi/abs/10.3109/15622975.2013.866693?journalCode=iwbp20. Acesso em: 7 ago. 2019.
JURUENA, Mario F; CLEARE, Anthony J; PARIANTE, Carmine M. The Hypothalamic Pituitary Adrenal axis, Glucocorticoid receptor function and relevance to depression. Rev. Bras. Psiquiatr. São Paulo, v. 26, n. 3, p. 189-201, Setembro 2004. Disponível em: <http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-44462004000300009&lng=en&nrm=iso>. Acesso em: 22/11/2019.
KAMINSKY, Z et al. Epigenetic and genetic variation at SKA2 predict suicidal behavior and post-traumatic stress disorder. Translational Psychiatry. Baltimore, p. 627-639. 25 ago. 2015. Disponível em: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4564560/>. Acesso em: 20/11/2019.
LABONTÉ, Benoit et al. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Translational Psychiatry. Montreal, p. 368-381. abr. 2014. Disponível em: <https://www.nature.com/articles/tp20143>. Acesso em: 31/10/2019.
MCNERNEY, M. Windy et al. Integration of neural and epigenetic contributions to posttraumatic stress symptoms: The role of hippocampal volume and glucocorticoid receptor gene methylation. Plos One. California, p. 421-434. 07 fev. 2018. Disponível em: <https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0192222>. Acesso em: 10 dez. 2019.
MULLIGAN, Connie et al. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. Florida, p. 853-857. jul. 2012. Disponível em: <https://www.tandfonline.com/doi/abs/10.4161/epi.21180>. Acesso em: 08 /12/2019.
NEUGEBAUER, Richard et al. Post-traumatic stress reactions among Rwandan children and adolescents in the early aftermath of genocide. International Journal Of Epidemiology. Nova Iorque, p. 1033-1045. ago. 2009. Disponível em: <https://academic.oup.com/ije/article/38/4/1033/849053>. Acesso em: 20 /12/ 2019.
PAQUETTE, Alison G. et al. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. Plos One. New Hampshire, 12 ago. 2014. p. 133-143. Disponível em: <https://www.ncbi.nlm.nih.gov/pubmed/25115650>. Acesso em: 20 out. 2019.
PERROUD, Nader et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. The World Journal Of Biological Psychiatry. Genebra, 01 abr. 2014. p. 334-345. Disponível em:
RADTKE, Karl M. et al. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational Psychiatry. Konstanz, p. 456-470. jul. 2011. Disponível em: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3309516/>. Acesso em: 20/10/2018
ROBERTS, Andrea L et al. Posttraumatic stress disorder across two generations: concordance and mechanisms in a population-based sample. Biol Psychiatry. Massachussetts, p. 505-511. abr. 2012. Disponível em: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3412195/>. Acesso em: 21/09/2019.
SCHECHTER, Daniel S. et al. The association of serotonin receptor 3A methylation with maternal violence exposure, neural activity, and child aggression. Behavioural Brain Research. Genebra, p. 268-277. 15 maio 2017. Disponível em: <https://www.sciencedirect.com/science/article/pii/S0166432816307720?via%3Dihub>. Acesso em: 20 out. 2019.
VUKOJEVIC, Vanja et al. Epigenetic Modification of the Glucocorticoid Receptor Gene Is Linked to Traumatic Memory and Post-Traumatic Stress Disorder Risk in Genocide Survivors. The Journal Of Neuroscience. Suiça, p. 10274-10284. 30 de julho 2014. Disponível em: http://www.jneurosci.org/content/34/31/10274.long. Acesso em: 02 nov. 2019.
YEHUDA, Rachel; BIERER, Linda M. Transgenerational transmission of cortisol and PTSD risk. Progress In Brain Research. Nova Iorque, p. 121-135. nov. 2007. Disponível em: <https://www.sciencedirect.com/science/article/pii/S0079612307670095?via%3Dihub>. Acesso em: 19/11/2019.
[1] Academic of the Medical Course of the Tiradentes University Center (UNIT-AL).
[2] Academic of the Medical Course of the Tiradentes University Center (UNIT-AL).
[3] Academic of the Medical Course of the Tiradentes University Center (UNIT-AL).
[4] Academic of the Medical Course of the Maurício de Nassau University Center (UNINASSAU).
[5] Professor, Master, Assistant Professor of the Medical Course of the Tiradentes University Center (UNIT-AL).
Sent: January, 2020.
Approved: August, 2020.















