REVIEW ARTICLE
NOVAES, Michele Franciene Baú [1]
NOVAES, Michele Franciene Baú. Evaluation of regulatory standards on labeling and packaging of pharmaceutical products. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 06, Vol. 01, pp. 44-64. June 2020. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/regulatory-standards
SUMMARY
The National Health Surveillance Agency (ANVISA) aims to promote the health of the population through the regulation of the production and marketing of products and services subject to sanitary surveillance, including pharmaceutical products. Regulatory standards guide the quality parameters that must be followed in the production chain in the pharmaceutical drug industry. This research sought to collect bibliographic data to clarify and make public information to health professionals, the pharmaceutical industrial sector, representative authorities linked to the Ministry of Health, ANVISA and the Brazilian population, in general, on the importance of discussing and updating Brazilian legislation on the labeling and packaging of pharmaceutical products, identifying and demonstrating data that indicate possible equivalences between them that may lead to medication errors , which may happen in exchange. It is also intended to verify with current sanitary standards what are the mandatory information to be inserted in the labels, relevant to user safety, and discuss about possible adverse reactions related to the lactose excipient, widely used by the pharmaceutical industry. To select the theoretical framework, we used the databases of the sites Scielo.br, VHL (Virtual Health Library), searches on websites, scientific journals and articles in health journals, where we used the search words alone: labeling, packaging, legislation, lactose, and combined search of two or three words: drug labels-medication errors, pharmacovigilance-medication errors, labeling-packaging-legislation, labels-packaging-lactose. The works that did not combine any of the three words were excluded, as well as those that did not fit in the pre-selected years from 2000 to 2019. The exclusion criterion also applies to articles that after reading that did not refer to the main objective of the research. There was a lack of specific standards to ensure the differentiation of similar packages to avoid medication errors, as was also noted the lack of rules to discriminate the presence of lactose on drug labels. The publication of legislation to promote packaging differentiation and the standardization of specific information on labels is fundamental for promoting the rational use of medicines.
Keywords: Drug labeling, packaging, medication errors. Legislation, lactose.
1. INTRODUCTION
The National Health Surveillance Agency (ANVISA) aims to promote the health of the population by monitoring the production and marketing of products and services submitted to sanitary surveillance, including pharmaceutical products, through inspection, standardization of procedures and processes to be followed in order to ensure quality and safety in the use of these products (BRASIL, 1999).
The industrial production chain of pharmaceutical products encompasses a number of sequential procedures aimed at ensuring quality, from pre-formulation studies to the development of labelling and suitable packaging material.
The quality of a pharmaceutical product must be observed from the processes of production of the assets to the label and packaging, which must meet all legal requirements.
ANVISA RESOLUTION RDC No. 71/2009 establishes the specifications, aspect and content that labels and packaging of medicines should be developed in Brazil, in order to facilitate access to the necessary information about these products and ensure the rational use of medicines (BRASIL, 2009).
The purpose of the packaging is to package, package, package, protect and maintain safe, and provide the proper conservation of the pharmaceutical product, constituting an important element in the efficacy and safety, in addition to conveying the essential information regarding the administration. On the labels must include the trade name of the product, if there is name of the active ingredient, the pharmaceutical form, route of administration, dosage and quantity packaged in the packaging material (BRASIL, 2009).
The Federal Constitution in one of its articles provides that health is guaranteed to all and it is the duty of the State to provide it, through socioeconomic policies that tend to reduce the likelihood of citizens developing diseases or other injuries, and access to actions and services for the promotion and protection of health of all Brazilian citizens (BRASIL, 1988).
The Consumer Protection Code provides that it is the consumer’s right to guarantee the protection of life and adequate and clear information on products and services. The products developed may not carry risks to the health or safety of consumers, and in the specific case of products produced in industrial environment, the manufacturer must provide all the necessary information and these must be accompanied by the product, especially information relating to the safety of use (BRASIL, 1990).
In 1998, the World Health Organization published the document “The Role of the Pharmacist in Self-Care and Self-Medication”, a report of the 4th WHO Advisory Group on the role of the pharmacist, a document that also reports the importance of access to impartial and quality information to guide self-care and self-medication (WHO, 1998).
Errors in dispensing and administering medications are derived from multifactorial consequences, having diverse origins and causes, from errors caused by dispensing and/or incorrect administration to those derived from unconscious self-medication of products containing substances that can generate problems or health problems of patients (WHO, 1998).
Scientific data indicate that labels and similar packaging of pharmaceutical products may be one of the reasons responsible for causing errors in dispensing and administering medicines in hospitals and health facilities (ANACLETO et al., 2005).
Other research shows that the use of some excipients in pharmaceutical preparations, widely used by industries in the manufacture of medicines, such as lactose, can cause the development of gastrointestinal discomforts in people with intolerance to this type of carbohydrate, both in the infant population and in adults when administering medications containing this excipient in preparation (SENA, 2012; SILVA, 2008).
Situations such as these can lead to hospitalization or increase the length of stay of hospitalized patients, bringing consequences to people’s health and the increase in costs spent for both public and private health systems.
We can consider occurrences such as those mentioned above as a public health problem to be analyzed, and we should discuss the laws involved in the process of labelling and packaging of pharmaceutical products, in order to guarantee the right to full health and information accessible and clear to the user.
Based on these occurrences, this research intends to evaluate the current regulatory standards in force in Brazil on the labeling and packaging of pharmaceutical products, analyze epidemiological data on medication errors derived from similar packaging, report the occurrence of adverse reactions derived from the use of drugs containing the lactose excipient, in order to verify the need for discussion and updating of Brazilian legislation on the subject , to facilitate and ensure the population’s access to essential information on medicines.
2. ANVISA’S ATTRIBUTIONS AND BRAZILIAN REGULATORY STANDARDS ON DRUG LABELING AND PACKAGING
The National Health Surveillance Agency, an agency linked to the Ministry of Health and characterized by administrative independence, has as one of its general attributions the responsibility for the standardization, control and supervision of products, substances and services related to health, act in health promotion and develop work in special situations that put at risk the health of the Brazilian population (BRASIL, 1999).
As specific attributions of this entity, we can mention the establishment of standards to regulate the drug sector, such as standardization of quality parameters for manufacturing; authorize and cancel registrations of inputs and pharmaceutical products; develop debates and public consultations on health-relevant issues to change or create new rules as a basis for activities in the areas of production and marketing of medicines; and analyze the requests of the pharmaceutical industries for changes in labels and packaging (BRASIL, 1999).
The development of civilization in a globalized world, the emergence of new pathologies to be investigated by medicine, and with the improvements achieved in recent decades in relation to the ease of access to medicines by the Brazilian population, compared to previous decades, there was an increase in the use of medicines and the search for better therapies to care for new diseases of modernity.
As a result of the demand for new, more effective and modern therapeutic alternatives, the need to develop new pharmaceutical inputs, design of pharmaceutical formulas and drug delivery systems was perceived. As a result, there has been an increase in investments by industries in research and development of new molecules, and as a result of this and the economic interests of the sector, there has been a considerable increase in the availability of drugs.
Drug labels and packaging have become not only an integral part of packaging the finished product, but for pharmaceutical marketing an important opportunity for the study and development of commercially attractive packaging that would help promote the product and increase sales. Then comes the need to establish sanitary standards for the labelling of these products.
Considering the criteria for Good Manufacturing Practices, which apply to all stages of research, production and logistics of a product, and the National Drug Policy instituted by the Ministry of Health in 1998, which has as one of its principles the incentive to the production of medicines and their sanitary regulation, the National Health Surveillance Agency published Resolution RDC No. 333/2003 (BRASIL, 2003; BRAZIL, 2019).
The Resolution of the Collegiate Board of ANVISA RDC No. 333/2003 was the first legislation developed regarding the labeling of medicines, establishing that all packages of medicines developed from the year following publication should adapt to the standards established by that resolution (BRASIL, 2003). Subsequently, this rule was repealed by the publication of Resolution RDC No. 71/2009.
Resolution RDC No. 71/2009 is the current norm in Brazil that regulates the characteristics that should be observed for the development of drug labeling in Brazil, in order to facilitate access to information and standardize the mandatory information to be entered (BRASIL, 2009).
Companies should notify the adequacy of the labeling, complying with the requirements set out in the resolution, and make new labels available on the packaging of medicines produced or imported for sale on the market within 540 days from the date of publication (BRASIL, 2009).
Some definitions are presented in this standard for types of labeling and their destination of use, and may have differentiated labeling and with characteristics that allow to be identified as a development label for use in products dispensed in public institutions linked to the Ministry of Health; commercial, with labeling developed for products with sales in pharmacies and drugstores; and hospital, with material intended to package products of restricted use for clinics and hospitals. The concepts for labeling and differentiation of packaging types are also characterized (BRASIL, 2009).
Label is all kind of identification printed or engraved on the labelling material that aims to identify important information in containers, containers, wrappers, wrappers or on the product in which it is desired to be identified (BRASIL, 2009).
Packaging is all kinds of form for packaging, with the main purpose of aggregating units of a product, aiming to create better conditions to maintain the quality and logistics distribution of the same (BRASIL, 2009).
When it comes to medicines, we still have the concepts of primary and secondary packaging. According to RDC No. 71/2009, primary packaging is the one that maintains direct contact with the pharmaceutical product, while the secondary packaging is the external packaging of the product, which is in contact with the primary packaging and may contain one or more primary packages (BRASIL, 2009).
According to the legislation mentioned above, there is information whose insertion becomes mandatory in the labeling process to clarify the characteristics of the drug.
On secondary packaging labels, they shall contain: the trade name of the medicinal product and its generic name of each active ingredient contained in the formulation, with the concentration of each of them; the route of administration, the pharmaceutical form, with indication of restriction of use by age group, indicating whether the product is intended for adult or pediatric use; the total quantity packed in the packaging material, with the indication of temperature and storage conditions; and information about the manufacturer and product registration data (BRASIL, 2009).
Warning phrases should also be inserted to the user in relation to contraindications, precaution and warning for the use of active ingredients, therapeutic class and excipients, when applicable, in secondary packaging. However, RDC No. 71/2009 does not specifically indicate which warning phrases regarding active and excipient ingredients should be included on labels and packaging (BRASIL, 2009).
With regard to primary packaging labels, it was found that the same mandatory information for secondary packaging should be inserted into the primary packaging and when it is not possible to contain all this information in the primary packaging material, industries must inform the reasons via electronic petitioning (BRASIL, 2009).
The labeling of medicines is the vehicle for communicating product information to the user. In this sense, the Brazilian regulatory agency has an important role to analyze and establish criteria through the publication of new normative acts for the development of labels and packaging appropriate to the needs of the population and more efficient from the information point of view.
3. LABELS AND PACKAGING OF SIMILAR MEDICINES AND MEDICATION ERRORS
Issues related to patient safety involve actions to prevent and eliminate errors involved in health processes. According to the United States National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP), medication error can be defined as:
any preventable event that may cause or lead to improper use of medications or harm to the patient while the drug is under the control of the healthcare professional, patient or consumer. Such events may be related to professional practice, health care products, procedures and systems, including prescription, communication, product label, packaging, nomenclature, composition, dispensation, distribution, administration, education, monitoring and use (NCCMERP, 2015).
According to the World Health Organization, medication errors are one of the main reasons for injuries and preventable damage in health systems, and can happen at different stages of the medication use process. Worldwide, the cost of drug-related errors is estimated at US$42 billion per year (WHO, 2017).
The publication of Law No. 13,236/2015 culminated in the amendment and inclusion of some articles of Law No. 6360/1976, to reinforce the need to distinguish labels and packaging of medicines in order to minimize the occurrence of medication errors, including errors arising from dispensing, administration of medicines or mistaken exchange for presenting similar packaging (BRASIL, 2015).
The labels and packaging of medicines, in addition to communicating the characteristics for recognition and use of these, has the purpose of being an important means to avoid errors. However, data point to medication errors in which labels and medication packaging can be the cause of confusion on the part of health professionals in the procedures performed in the work environment, and consequently harm the health of patients (ANACLETO et al., 2005).
Medication errors are a major public health problem and are responsible for the occurrence of morbidity, hospitalizations, mortality and increased health expenditures. According to a publication in 2018 by the Institute for Safe Practices for the Use of Medicines, among the errors related to medicines, 33% are caused by labels and packaging, and 31% of these have evolved to related fatalities (ISMP, 2018).
An epidemiological study of the observational and cross-sectional type was carried out in 2010 in different sectors of the hospital pharmacy of a university hospital in Fortaleza, Ceará, with the purpose of verifying labels and packaging of medicines, identifying similarities in them that could trigger medication errors that could occur in the processes of storage, dispensing and administration (LOPES et al., 2012).
We examined 300 pharmaceutical presentations photographed (150 pairs) and presented to observers on different days, and the observers participating in the study were two nurses and a pharmacist (LOPES et al., 2012).
The pharmaceutical presentations of the study sample included reference drugs, generics, similar drugs and drugs with packaging from the Ministry of Health. Pharmaceutical forms and presentations, types of packaging, name, color, design, pharmacological class, suppliers and risk potential were analyzed; considered potentially dangerous medicines and controlled medicines (LOPES et al., 2012).
For the selection of samples, reference drugs, generics, similar drugs and drugs with packaging from the Ministry of Health were considered. In the occurrence of similarities, the data were entered into a database and characterized from international references such as the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP, 2017).
After presenting the photos to the observers, collecting the answers obtained by them and analyzing the data using statistical methods, the researchers found the following results: regarding the type of medication, 43% of similar packages were of similar drugs, 21.33% of reference drugs and 18% of generic drugs; and, among the 24 pharmacological classes, 28% were antimicrobials, 10.67% anesthetics and 6.67% antihypertensive (LOPES et al., 2012).
Similarities were also found regarding the printed data, the designer, color of the labels, packaging with the same color. The researchers identified that; among the sample analyzed, 50% of the primary packages and 44% of the secondary packages had the same color (LOPES et al., 2012).
The investigation of the pharmaceutical presentations studied in this research also showed that, in the total of possibly similar drugs, 51.33% were small-volume parenteral solutions including ampoules, vials and closed-system bags, demonstrating the influence that the theme of labeling and packaging of medications has on the ability to enable medication errors (LOPES et al., 2012).
As a representation of the observation problem presented in this research, we tried to identify for example some labels and similar packaging of medicines. Some images were found from searching on websites.
The Ministry of Health in 2016 updated the Manual of Visual Identity of Drug Packaging, which established the criteria for the development and identification of the labeling of medicines distributed by the Unified Health System.
Figure 1 shows injectable drugs produced and distributed by the Ministry of Health for use in the UNIFIED HEALTH SYSTEM (SUS) and represents the similarity in the labeling of two different types of insulins, the possible exchange of which may put the patient at risk if there is erroneous administration of regular human insulin (packaging presented on the left side of the image) instead of human insulin NPH (presented on the right side of the image).
Figure 2 presents similar packages of drugs with controlled substances that are classified as anticonvulsants, commonly used to prevent the triggering of seizures in patients with epilepsy. The manufacturer’s name engraved on the bottom of the package was purposely covered with black marked so as not to expose the mark and avoid possible questions regarding the interests of this research.
Figure 1. Similar insulin packaging and the potential for medication error.

Figure 2. Similar packages of anticonvulsants manufactured in Brazil.

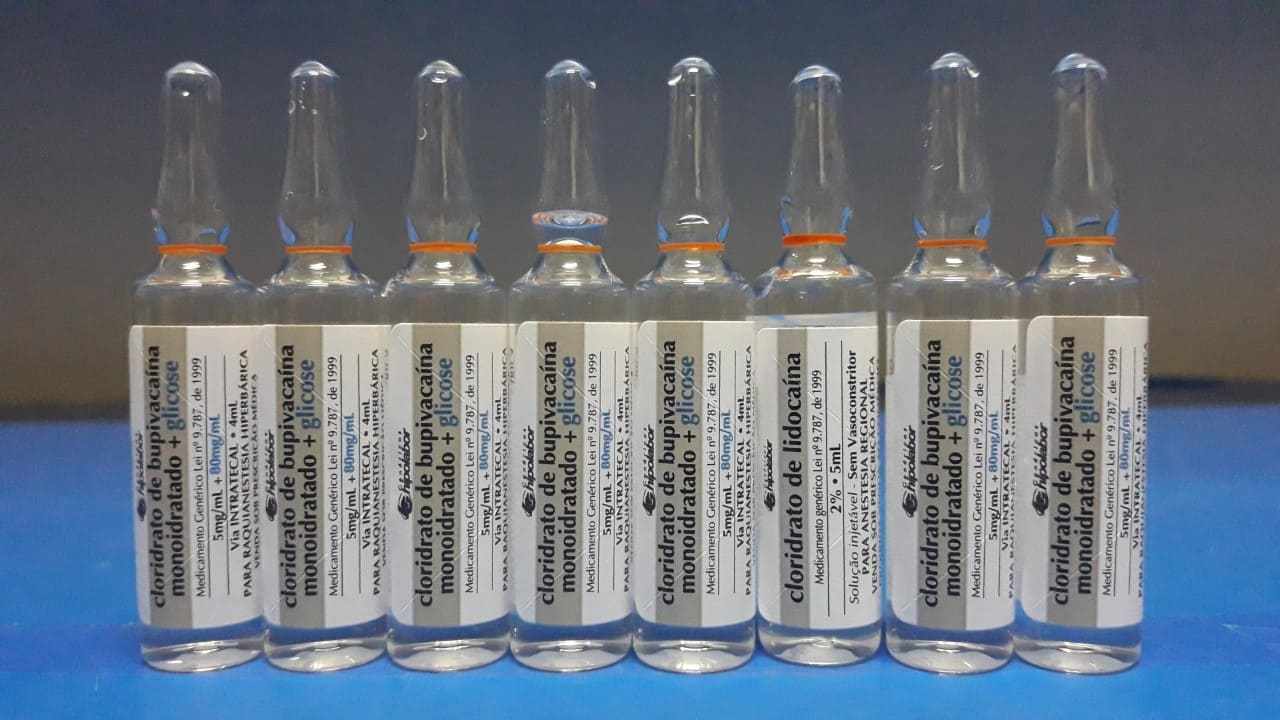
Another similarity in the labeling of medicines was identified by the author of this research while he was responsible for providing pharmaceutical assistance in a hospital in the city of Votorantim, in the state of São Paulo, Brazil, from the observation of the pharmacy stock of the operating room, in the boxes (called “psychobox”) intended for use by anesthesiologists in the operating rooms, where the team reported the occurrence of administration error between lidocaine and bupivacaine.
The ampoules of injectable drugs of lidocaine and bupivacaine found in the boxes were analyzed and presented similarity potential to induce medication error, both for dispensing and administration in patients in surgical procedures.
It is noteworthy that the ampoules of these two substances have the same company as manufacturer and for the purpose of observing the similarity were placed, empirically, several of these ampoules side by side with the introduction of an ampoule of different substance among the others for analysis of the potential for similarity. Figures 3 and 4 below present the situation described.
Figure 3. Injectable drugs used in surgical procedures and aspects of labeling.

Figure 4. Enlarged photo. Injectable drugs used in surgical procedures and aspects of labeling.
Another research published in the journal of the Israeli Institute of Teaching and Research Albert Einstein, sought to demonstrate that colors help in the process of visual identification of eye drops, reducing the incorrect administration of eye drops. The labels of the packaging of the tested products were removed and the identification of eye drops was encouraged by patients from the characteristic color of each presentation. The researchers concluded that staining of the drug substance can help in the process of visualization and identification of medications (COSTA et al., 2019).
From this research and the data presented earlier, empirically, we can understand that colors interfere in the identification of medicines, both in the active substance present, as well as could positively influence the labeling and packaging process of pharmaceutical products.
Different colors being used in the processes of development of packaging material to improve the distinction of labeling, such as ampoules, vials, bags of parenteral solutions, glassware, blisters, among others, decreased the possibility errors per exchange.
4. THE USE OF LACTOSE AS A PHARMACEUTICAL EXCIPIENT
In the studies for the design of new pharmaceutical formulas, the biopharmaceutical and physicochemical characteristics of the active and excipient ingredients are analyzed. Pharmaceutical forms require adjuvants to assist in processes such as solubility, compression, conserve, give volume, shape to the drug and give pleasant flavor to the formulation to improve consumer acceptance (AULTON, 2016).
Lactose is a disaccharide carbohydrate, being one of the types of excipient commonly used by the industry, and which presents itself as diluent and sweetener, especially in solid pharmaceutical forms, and may also interfere in pharmacokinetic characteristics. A contraindication of the use of this excipient in pharmaceutical preparations would be the fact that some people have lactose intolerance (AULTON, 2016; 1475).
Lactose intolerance is a condition of the organism where there is the inability of digestion of this carbohydrate due to deficiency of the digestive enzyme lactase, which transforms lactose into glucose and galactose, being a condition that causes life problems of people worldwide (PEREIRA; FERREIRA; MARQUES, 2019).
People who are present with this condition, suffer from gastrointestinal discomforts, such as the development of prolonged severe diarrhea, flatulence, cramps, abdominal swelling and dehydration, which leads them not to consume milk and other dairy products (PERREIRA; FERREIRA; MASQUES, 2019).
Patients with lactose intolerance are subject to adverse reactions resulting from the use of medications containing this excipient, and may develop symptoms similar to those that occur when consuming foods containing this sugar, since the intake of the amount equal to or less than 3 grams can already provoke the described symptoms (SENA et al., 2014).
A study described conducted at the Pharmacovigilance Center of Ceará aimed to identify the existence of possible excipients that induce adverse reactions contained in 12 products (35 pharmaceutical presentations) with the highest sale in Brazil, and the sample was collected from August to September 2004 (SILVA et al., 2008).
Lactose was present among the pharmaceutical adjuvants found in the products studied, such as in the formulation containing bromazepam (controlled substance whose acquisition requires specific prescription) and dipyrone associated with butylschopolamine (SILVA et al., 2008).
Another descriptive study with a bibliographic survey of the composition of medicines, through the package leaflet and the technical information recorded in ANVISA, evaluated the risk of possible excipients that cause adverse reactions in contraceptive medications. The sample was characterized by 6 contraceptive presentations, 3 of which were reference drugs and 3 were similar, and in all of them the presence of lactose in the composition was found (DE CASTRO et al., 2010).
Research conducted with drugs often prescribed to pediatric patients also analyzed the presence of lactose in 181 different presentations of 42 drugs. The pharmacological groups found were: analgesics/antipyretics, nonsteroidal anti-inflammatory drugs, antibiotics, antihistamines, antiemetics, oral corticosteroids, inhaled corticosteroids, long-acting bronchodilators, inhaled corticosteroids associated with bronchodilators, antileukotrienes and mast cell membrane stabilizers. Regarding the presence of lactose, 28% of the presentations contained this excipient (GERMANA, et al. 2009).
The verified studies show data that identify the presence of lactose in a considerable percentage among the products analyzed. Currently in Brazil there is no legislation that regulates drug manufacturers to add on the outside of the package the information that the drug presents the excipient lactose.
Secondary drug packaging is in direct contact with the product and are commonly identified by the lay population in pharmaceutical legislation as the external and first visually identified: “the medicine box”. Considering that other warning information about dyes has already been inserted in the labeling of medicines through legislation, such as tartazine, it would be interesting to consider inserting the alert also on lactose on the outside, facilitating identification.
Labeling could help people with lactose intolerance to make better decisions about the choice and purchase of their medicines. A bill submitted to the House of Representatives in 2009, it provided for requiring pharmaceutical laboratories to insert on drug labels warning about the existence of lactose in the composition of their products. Although the theme of the bill is relevant for the safe and rational use of medicines, the proposal was analyzed and filed in 2013.
5. THE RIGHT TO ACCESS TO HEALTH INFORMATION AND THE LABELLING OF MEDICINAL PRODUCTS
Health is the right of all and the duty of the State, guaranteed through social and economic policies aimed at reducing the risk of disease and other injuries, and universal and equal access to actions and services for its promotion, protection and recovery (BRASIL, 1988).
The right to health is inserted within the scope of constitutionally guaranteed social rights (BRASIL, 1988). Based on the Federal Constitution as a principle to highlight the right to health and information derived from this right, it is up to the Brazilian regulatory agency as an integral and representative part of the Ministry of Health, the responsibility for taking initiatives that will develop and ensure access to the population on the health issues that are relevant to them.
The Organic Health Law No. 8080/1990, that creates the Unified Health System, resumes this statement contained in the Brazilian Federal Constitution, reaffirming that health is a fundamental right of the human being and the duty of the State to provide the fundamental circumstances for its full exercise, and the State must also guarantee health by establishing conditions that ensure access, but makes it clear that the duty of the State does not exempt the responsibility of people, the family, companies and society in matters related to health. (BRAZIL, 1990).
In a hospital environment, medicines are commonly stored in the pharmacy and dispensing is performed by professionals working in the sector, with the pharmacist being the team member responsible for all activities developed within the hospital pharmacy. The visually distinct labeling of other products allows the correct identification of the drug at the time of dispensing and prevents errors in the administration of medicines by health professionals.
In pharmacies and drugstores, medicines can be purchased by the user himself or another person requested by the user, and can be purchased medicines made available in the self-service and known as MIPs (Non-Prescription Drugs), drugs that need to be prescribed by a health professional qualified for this purpose, including controlled drugs, the prescription of which will be retained in the establishment dispensing the product.
In the case of adverse reactions caused by patients with lactose intolerance, for example, the right to access health information becomes essential. Considering that many medicines contain lactose as excipient, including controlled medications, if the user comes to develop an adverse reaction to the drug because it contains lactose, this product will probably be unusable and the patient will need to ask the prescriber for another prescription to make a new purchase. Such cases could be avoided with warning phrases on the packaging, since the information about excipients is inserted only in bullium.
Medications are available in different environments, but are used for the same purpose of preventing, curing, relieving symptoms or assisting in the diagnosis of diseases. Understanding these premises, we can realize that the information about these products must reach users efficiently, in order to provide safe use and without additional risks, in addition to those known and already manifested in the package leaflet.
Information about products, especially those related to health, should be adequate and clear in order to accurately present all the evidence that guarantees safety regarding use, as provided for in Article 6 of the Consumer Protection Code (BRASIL, 1990).
Health regulations are the way to establish and guarantee this right to consumers of pharmaceutical products. Furthermore, the pharmaceutical industry would have much to gain from the provision of more efficient information on labeling, in addition to those required by current legislation, since products that add value to consumer safety and prescription safety by health professionals prescribing medicines would have a greater possibility of prescription and sale.
Industrial development of packaging material and the right to health information should be interlinked. Considering that health professionals often work long working hours a day, similar packaging poses a risk to erroneous administration and do not collaborate to meet the safety criteria in the use of medications.
Changes in the labeling and packaging of medications could minimize the occurrence of medication errors, alert people with lactose-related problems about adverse reactions and promote the rational use of medications, ensuring direct health and health information.
6. FINAL CONSIDERATIONS
After analyzing the sanitary standards on the labelling and packaging of pharmaceutical products, it was possible to find generic concepts and recommendations for the development of labelling and the lack of specific rules was observed to inhibit possible medication errors derived from labels and similar packaging.
At the same time, no sanitary standard found the determination by the Brazilian regulatory agency on the obligation for pharmaceutical industries to insert the information: ‘CONTAINS LACTOSE’, in the packaging and labels of medicines, specifying to health professionals and patients about the composition of the pharmaceutical formula directly in the secondary packaging: “outside of the box”.
The costs arising for health systems worldwide generated by medication errors and adverse reactions are high, and the consequences of the similarity and lack of information on excipients in labels and packaging contribute to increased spending and raise the possibility of risk to the health of all.
Medication errors and adverse events derived from the labeling and packaging of pharmaceutical products need to be investigated by public health authorities and ANVISA, an agency linked to the Ministry of Health, in order to seek solutions to the issue highlighted in this research, and try to find ways to favor the rational use of medicines aligned with the development by the pharmaceutical industries of labels and packaging more informative than commercial.
The occurrence of this situation apparently has not moved the pharmaceutical industries to develop projects in order to create new solutions, providing the standardization of packaging and labels of medicines, forgetting that the quality parameters to meet good manufacturing practices should be observed from the studies of development and production of pharmaceutical forms to the control of quality and safety in post-marketing use.
Simple actions of the drug industry could improve the lives of patients and health professionals in drug management. The use of different colors to differentiate drug labeling, especially those with moderate to severe drug interaction potential, and the insertion of a specific warning phrase for lactose products directly on the outside in drug packaging, could avoid errors and facilitate access to relevant information.
This research hopes to contribute to further studies being developed, and measures are taken on the subject, both by public institutions and the pharmaceutical industrial sector, in order to provide that debates occur and solutions can be found.
It is first up to the National Health Surveillance Agency, as the entity responsible for developing health promotion, the role of reanalyzing the current legislation on labeling and packaging of medicines and publishing new normative acts that solve the issues presented in this research.
Labels and packaging have been the cause of medication errors and related adverse reactions. Health regulation is the way to provide the prevention and minimization of drug-related problems.
7. REFERENCES
ANACLETO, Tânia Azevedo et al. Erros de medicação e sistemas de dispensação de medicamentos em farmácia hospitalar. Clinics. São Paulo, v. 60, n. 4, p. 325-332, ago. 2005. Disponível em: <http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1807-59322005000400011&lng=pt&nrm=iso>. Acesso em: 29 nov. 2019. http://dx.doi.org/10.1590/S1807-59322005000400011.
ANVISA, 2019. Registro de medicamentos cresce 100%. Disponível em: <http://portal.anvisa.gov.br/noticias/-/asset_publisher/FXrpx9qY7FbU/content/registro-de-medicamentos-cresce-100-em-2019/219201?p_p_auth=fJVL8wqt&inheritRedirect=false>. Acesso em: 06 jan. 2020.
ARAUJO, Ana Carolina; BORIN, Maria de Fátima. Influência de excipientes farmacêuticos em reações adversas a medicamentos. Brasília Med. 2012;49(4):267-278. Disponível em: <https://pdfs.semanticscholar.org/e5a3/9e36b11ae4dc9336d3bd033fe80cd45f2df1.pdf>. Acesso em: 15 jan. 2020.
AULTON, Michael E. Aulton delineamento de formas farmacêuticas. Michael E. Aulton, Kevin M. G. Taylor; [tradução Francisco Sandro Menezes]. Versão digital – 4 ed. – Rio de Janeiro: Elsevier, 2016.
BRASIL, 1988. Constituição da República Federativa do Brasil. Disponível em: <http://www.planalto.gov.br/ccivil_03/constituicao/constituicao.htm>. Acesso em: 20 nov. 2019
BRASIL, 1990. Lei nº 8.078, de 11 de setembro de 1990. Dispõe sobre o Código de Defesa do Consumidor. Disponível em: <http://www.planalto.gov.br/ccivil_03/leis/l8078.htm>. Acesso em 17 dez. 2019.
BRASIL, 1999. Lei nº 9.782, de 26 de janeiro de 1999. Disponível em: <http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2006/prt0354_11_08_2006.html>. Acesso em: 15 de jan. de 2020.
BRASIL, 2016. Manual de Identidade Visual de Medicamentos do Ministério da Saúde. Disponível em: < https://www.saude.gov.br/artigos/672-assuntos/assistencia-farmaceutica/42827-arquivos-do-manual-de-identidade-visual-de-medicamentos>. Acesso em: 22 jan 2020.
BRASIL, 2003. Resolução RDC nº 333, de 19 de novembro de 2003. Disponível em: <http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2003/rdc0333_19_11_2003.html>. Acesso em: 27 out. 2019.
BRASIL, 2019. Resolução RDC nº 71, de 22 de dezembro de 2009. Disponível em: <http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2009/res0071_22_12_2009.html>. Acesso em: 23 nov. 2019.
BRASIL, 2015. Lei nº 13.236, de 29 de dezembro de 2015. Disponível em: <https://www2.camara.leg.br/legin/fed/lei/2015/lei-13236-29-dezembro-2015-782195-publicacaooriginal-149108-pl.html>. Acesso em: 27 out. 2019.
BRASIL, 2019. Resolução RDC nº 301, de 21 de agosto de 2019. Disponível em: <http://www.in.gov.br/web/dou/-/resolucao-rdc-n-301-de-21-de-agosto-de-2019-211914064>. Acesso em 14 dez. 2020.
COSTA, Ana Luiza Fontes de Azevedo et al. Quando a cor ajuda. Einstein (São Paulo), São Paulo, v. 17, n. 1, eAO4410, 2019. Disponível em <http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1679-45082019000100200&lng=en&nrm=iso>. Acesso em 29 nov. 2019. Epub Dez 17, 2018. http://dx.doi.org/10.31744/einstein_journal/2019ao4410.
DE CASTRO, Ana Luiza Ferreira Meinen; AHLERT, Elias Ricardo; COLET, Christiane de Fátima. Avaliação do risco de reações adversas causadas por excipientes em formulações de anticoncepcionais. Revista Contexto & Saúde, v. 10, n. 19, p. 146-149, 14 jun. 2013. Disponível em: <https://www.revistas.unijui.edu.br/index.php/contextoesaude/article/view/1496>. Acesso em: 15 nov. 2019.
GERMANA, Stefani P. et al. Presença de corantes e lactose em medicamentos: avaliação e 181 produtos. Rev. bras. alergia imunopatol; v. 32, v.1, p. 18-26, jan.-fev. 2009. Disponível em: < http://www.sbai.org.br/revistas/Vol321/ART%201-09%20-%20Presen%C3%A7a%20de%20corantes%20e%20lactose.pdf>. Acesso em: 15 jan. 2020.
ISMP, 2018. Estratégias de segurança do paciente no Brasil – O que já foi feito e o que ainda está por vir?. Disponível em: <https://www.ismp-brasil.org/site/wp-content/uploads/2019/05/A—-es-da-Anvisa-com-foco-seguran–a.pdf>. Acesso em: 22 jan. 2020.
LOPES, Diana Maria de Almeida et al. Análise da rotulagem de medicamentos semelhantes: potenciais erros de medicação. Rev. Assoc. Med. Bras., São Paulo, v. 58, n. 1, p. 95-103, fev. 2012. Disponível em <http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-42302012000100021&lng=en&nrm=iso>. Acesso em: 29 nov. 2019. http://dx.doi.org/10.1590/S0104-42302012000100021.
National Coordinating Council for Medication Error Reporting and Prevention. What is a medication error? Nova York: National Coordinating Council for Medication Error Reporting and Prevention; 2015. Disponível em <http://www.nccmerp.org/aboutmedication-errors>. Acesso em 20 de jan. 2020.
PEREIRA, Leandra Gonçalves; FERREIRA, Michelle Silva; MARQUES, Fabíola Pedrosa Peixoto, 2019. Intolerância à lactose e os aspectos legais da rotulagem. Disponível em: <http://anais.unievangelica.edu.br/index.php/latosensu/article/view/4526>. Acesso em 15 jan. 2020
PESSANHA, Ana Flávia de Vasconcelos et al. Influência dos excipientes multifuncionais no desempenho dos fármacos em formas farmacêuticas. Rev. Bras. Farm. 93(2): 136-145, 2012. Disponível em: <http://www.rbfarma.org.br/files/rbf-2012-93-2-2.pdf>. Acesso em: 15 jan. 2020
SENA, Cristina Santana et al. Excipientes farmacêuticos e seus riscos à saúde: uma revisão da literatura. Rev. Bras. Farm. 93(2): 136-145, 2014. Disponível em: <http://www.sbrafh.org.br/v1/public/artigos/2014050405000621BR.pdf>. Acesso em: 15 jan. 2020
SILVA, Antonio Vinicios Alves da et al. Presença de excipientes com potencial para indução de reações adversas em medicamentos comercializados no Brasil. Rev. Bras. Cienc. Farm., São Paulo, v. 44, n. 3, p. 397-405, set. 2008. Available from <http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-93322008000300009&lng=en&nrm=iso>. Acesso em: 29 nov. 2019
WHO, 1998. Portal de informações sobre medicamentos essenciais e produtos de saúde. Relatório do 4º Grupo Consultivo da OMS sobre o papel do farmacêutico. Disponível em: <https://apps.who.int/medicinedocs/en/d/Jwhozip32e/>. Acesso em: 15 jan. 2020.
WHO, 2017. O terceiro desafio global da segurança do paciente da OMS: medicação sem danos. Disponível em: <https://www.who.int/patientsafety/medication-safety/campaign/en/>. Acesso em 06 jan. 2020.
[1] Postgraduate in Pharmaceutical Industrial Management from Estácio de Sá University, Graduated in Pharmacy-Biochemistry from Universidade Paulista – UNIP.
Sent: March, 2020.
Approved: June, 2020.















