REVIEW ARTICLE
TAVARES, Marcela Marçolla [1]
TAVARES, Marcela Marçolla. Use of Colloids and Crystalloids in the perioperative period of severe patients: Controversias. Multidisciplinary Core scientific journal of knowledge. Year 05, Ed. 01, Vol. 01, pp. 05-21. January 2020. ISSN: 2448-0959, Access link in: https://www.nucleodoconhecimento.com.br/health/colloid-and-crystalloids
SUMMARY
As the management of fluid replacement influences the patient’s prognosis, basic and clinical research has addressed the various different aspects that contribute to the administration of fluids and volume in the perioperative period. There is still no consensus on when and in whom to indicate perioperative hemodynamic optimization. Additional issues such as the relative role of crystalloid versus colloid ratio optimization were not resolved. This work consists of a literature review, conducted between February and October 2019, in which a consultation was made to various scientific publications, present on the Internet, through consultation in the databases: ScienceDirect, UptoDate, Medline/PubMed, Lilacs, sciELO, with the terms Perioperative Fluid therapy, Hemodynamics, Volume Reposition, Crystalloid, Colloid. From this, it was observed that fluids should be applied like any other medication, respecting their individual indications and limitations, which is the basis of the concept of Goal-Guided Therapy – a new concept that encourages the patient’s framing of inclusion criteria for fluid optimization. Referring to this, advantageous aspects of perioperative volume replacement with iso-oncotic solutions are understandable, although additional randomized controlled trials are needed to prove its relevance to the outcome.
Keywords: Perioperative Fluid Therapy, Hemodynamics, Volume Replacement.
1. INTRODUCTION
As the management of fluid replacement influences the prognosis of the patient, basic and clinical research has addressed the various different aspects that contribute to the administration of fluids and volume in the perioperative period. The basic research improved the knowledge about the function of the endothelial vascular barrier and its functional alterations responsible for vascular extravasation. Clinical studies proclaiming different approaches to fluid control have shown conflicting results and, in most cases, do not refer to the physiological basis of the vascular barrier. Based on the meta-analysis of the last 30 years, there have been a series of studies conducted to reduce mortality and surgical morbidity by deliberate and preventive manipulation of perioperative hemodynamics, evidencing a tendency of the scientific community to clarify whether hemodynamic optimization is indeed beneficial[2].
It is responsible for studies that focused mainly on clinical objectives to guide volumetric therapy in the perioperative period. However, a reasoning should generally be derived from physiological facts and significant and comparable studies. Thus, this review summarizes the relevant knowledge about the effect of different intravenous fluids and on hemodynamic monitoring. As there is still no consensus on this subject of great interest to all anesthesiologists, the aim of this article is to clarify some advantages and disadvantages of the main fluids used in perioperative volume replacement.
The relative role of optimizing the crystalloid versus colloid relationship, although not resolved, there is evidence that favors balanced solutions with an electrolyte concentration similar to that found in plasma, and large volumes of saline solution should be avoided, as they induce hyperchloremic acidosis[3].
2. MATERIAL AND METHODS
This work consists of a literature review, conducted between February and October 2019, in which a consultation was made to various scientific publications, present on the Internet, through a search, from the databases of ScienceDirect, UptoDate, Medline/PubMed, Lilacs, sciELO, mainly with the terms Perioperative Fluid therapy, Hemodynamics, Volemic Reposition, Crystalloid, Colloid.
The criteria for inclusion in this study were scientific articles describing the main differences between colloids and crystalloids as well as their main indications in the perioperative period. Several modalities of studies between 1980 and 2019 were reviewed, including randomized and multicenter prospective studies, meta-analyses, retrospective observational studies and literature reviews.
3. REVIEW
3.1 THE PHYSIOLOGY OF FLUID COMPARTMENTALIZATION
The total body water for an individual of 75 kg is approximately 45 L (60%). Two thirds of this (30 L) is intracellular water. The remaining third (15 L) in the extracellular compartment is divided between the intravascular (3 L) and extravascular (12 L) compartments (Fig. 1[4]). The total intravascular volume is approximately 5 L and has cellular components (red and white blood cells and platelets: 40%[2 L]) and non-cellular (plasma: 60%[3 L]). Plasma is an aqueous solution of inorganic ions (predominantly sodium chloride), simple molecules such as urea, and larger organic molecules such as albumin and globulins.
FIGURE 01: Explanatory model of fluid distribution in the body
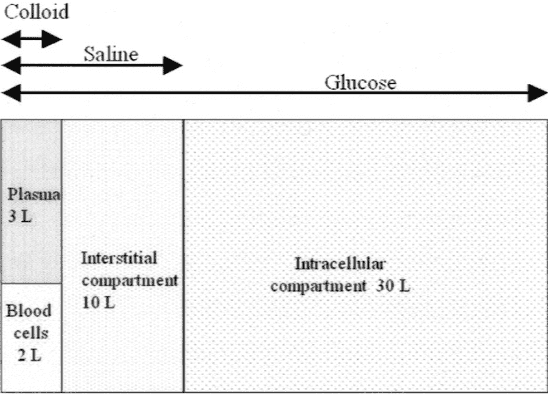
As shown in Figure 01, it is noted that a large part of the liquid volume is concentrated in the intracellular medium, being composed of glycosate solution.
Increased vascular permeability due to inflammatory activation with impaired endothelial cell function may occur for a variety of reasons during larger surgical procedures[5]. Specifically, surgical tissue trauma, tissue hypoperfusion due to inadequate fluid therapy, ischemia injury/reperfusion, sepsis (local or blood) and use of cardiopulmonary bypass (e.g., cardiopulmonary bypass) are recognized as inflammatory stimuli that may compromise vascular function[6].
3.2 VOLUME REPLACEMENT
Volume replacement should be indicated whenever there is a need to: 1) replace blood loss during surgery, 2) Replace losses with sweating and urine production 3) Preoperative bowel surgeries and 4) maintenance of third space losses[7].
It is understood by third space to be a term proposed by Randall in 1952, to describe the situation in which extracellular fluid is lost or kidnapped in an area of the body where it does not participate in the exchanges, and consequently does not meet the patient’s water needs[8].
3.3 PERIOPERATIVE HEMODYNAMICALLY OPTIMIZED STRATEGY
The patients who could benefit from this therapy are those at high risk according to the Revised Cardiac Risk Index and the Preoperative Cardiopulmonary Exercise test[6],[9],[10]
According to a systematic literature review on perioperative optimization in patients at high surgical risk, important factors were found that would help identify patients who would benefit most from perioparatory fluid optimization[11]. The results are described in Table 01, based on the studies by Mangano et al. (1996), Poldermans et al. (1999) and Shoemaker et al. (1988).
TABLE 01 – Inclusion criteria for perioperative optimization
| Histórico de enfermidade cardíaca ou pulmonar severa, como por exemplo DPOC, infarto agudo do miocárdio, e insuficiência cardíaca. |
| Cirurgias extensas que envolvam anastomoses do trato gastrointestinal (por exemplo, esofagectomia). |
| Perda massiva e abrupta de sangue (>2,5L) |
| Idade maior ou igual a 70 anos com reserve fisiológica limitada em um ou mais órgãos vitais. |
| Septicemia |
| Insuficiência Respiratória (PaO2 <60 mmHg e FIO2 >0.4) |
| Acometimento abdominal agudo como pancreatite, perfuração de vísceras ou sangramentos do TGI. |
| Insuficiência Renal Aguda |
| Doença vascular em estágio avançado |
Source: Tote; Grounds (2006)
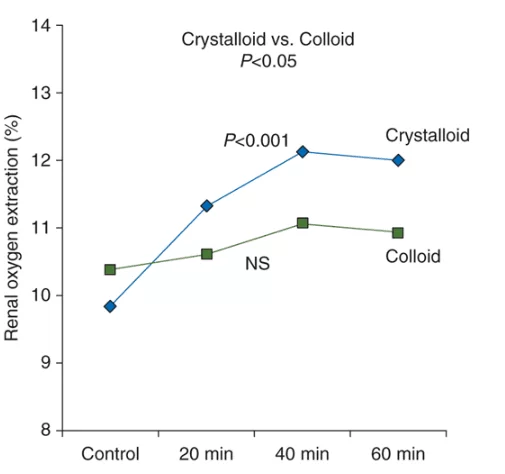
Based on a study involving 30 severe patients who underwent cardiac surgeries, it was evidenced that despite the increase in cardiac index and renal blood flow with both fluids, none of the fluids improved renal oxygenation, because both induced hemodilution. GFR increased in the crystalloid (28%), but not in the colloid group, as shown in Figure 02[12].
The same study showed that the use of crystalloids increased the filtration rate (24%) as well as renal oxygen extraction (23%), even without increasing the vascular supply of the component, suggesting that the increase in GFR – the main determinant of oxygen consumption – was not accompanied by a proportional increase in the fraction of oxygen supplied to the kidneys. In other words, their results summarize in graph (figure 02) that the crystalloid increased renal oxygen extraction (P <0.001) 20, 40 and 60 min after bolus, in contrast to colloid (NS), suggesting impaired renal oxygenation in the second case. The change in renal oxygen extraction was significantly (P <0.05) more pronounced in the crystalloid group compared to the colloid group[11].
FIGURE 02: Effects of crystalloid (10 ml/kg) and colloid infusion (20 ml/kg) on renal oxygen extraction after cardiac surgery
3.4 THE TYPE OF FLUID FOR HEMODYNAMIC OPTIMIZATION STRATEGY
Numerous presentations of solutions are available for volume replacement, they vary according to the composition of ions and the presence or absence of organic compounds. So we have the presence of colloids and crystalloids.
Colloids are considered plasma expanders because they cause less capillary loss and less pulmonary edema than crystalloids. They reduce the expression of inflammatory mediators, improve tissue microcirculation and oxygenation, and promote higher volume resuscitation than crystalloids. However, side effects (CS) have already been observed[5].
The crystalloids, on the other hand, leave the intravascular compartment earlier and in greater quantity than colloids and, therefore, a larger volume is necessary to replenish deficits (3 to 4 times the volume of crystalloids[6]). Some of the main characteristics of crystalloids are presented in tables 02 and colloids in table 03[3].
TABLE 2 – Main crystalloids and their osmolarities and concentrations of Sodium (Na+) and Potassium (K+)
| Cristaloide | Osmolaridade (mOsm/L) | Na+ | K+ |
| Glucose 5% | 252 | — | — |
| Glucose 25% | 1260 | — | — |
| Glucose 50% | 2520 | — | — |
| Sodium chloride 0.9% | 308 | 154.0 | 154.0 |
| Sodium chloride and glucose | 264 | 31.0 | 31.0 |
| Ringer’s solution | 309 | 147.0 | 156.0 |
| Compound sodium lactatea | 278 | 131.0 | 111.0 |
| Plasmalyte B | 298.5 | 140 | 98 |
| Normasol | 280 | 140 | 98 |
Source: Adapted from Grocott. Et al (2005)
In a prospective, blind study in elective patients who had non-cardiac surgery, intraoperative fluid resuscitation with colloid use (6% HES), the incidence of postoperative nausea and vomiting, severe pain, periorbital edema and double vision was reduced[13].
In patients undergoing major abdominal surgery, when they received crystalloids alone, they resulted in intestinal fluid accumulation compared to those who also received colloids (6% HES)[14]. This leads to increased tissue edema. The association of tissue edema with impaired perfusion and oxygenation is controversial[15]
TABLE 3: Main colloids and their respective values of Weighted Average Molecular Weight (PMMP), Weighted Average Molecular Number (NMMP) and Sodium (Na+) and Chlorine (Cl-) concentrations
| Solução | Coloide | PMMP(Da) | NMMP (Da) | Na+(mmol/L) | Cl- (mmol/L) |
| Gelofusine (4%) | Succinylated gelatin | 30,000 | 22,600 | 154 | 125 |
| Haemaccel (3.5%) | Polygeline | 35,000 | 24,300 | 145 | 145 |
| Voluven | Tetrastarch | 130,000 | 60,000 | 154 | 154 |
| Pentaspan | Pentastarch | 264,000 | 63,000 | 154 | 154 |
| HAES-steril 6% or 10% | Pentastarch | 200,000 | 60,000 | 154 | 154 |
| EloHase 6% | Hexastarch | 200,000 | 60,000 | 154 | 154 |
| Hespan 6% | Hetastarch | 450,000 | 70,000 | 150 | 150 |
| Hextend | Hetastarch | 670,000 | 70,000 | 143 | 124 |
| Gentran 40 | Dextran 40 | 40,000 | 25,000 | 154 | 154 |
| Gentran 70 | Dextran 70 | 70,000 | 39,000 | 154 | 154 |
| Rheomacrodex | Dextran 40 | 40,000 | 25,000 | 154 | 154 |
| Macrodex | Dextran 70 | 70,000 | 39,000 | 154 | 154 |
Adapted from Grocott; Mythen, mythen, mythen. GAN (2005)
On the other hand, large amounts of saline solution lead to hyperchloremic acidosi[16]s and the state of hypercoagulobilit[17]y even more evidenced than that normally seen in the infusion of other crystalloids[18].
3.5 RATIONAL USE OF FLUIDS IN THE PERIOPERATIVE PERIOD
More than the type of fluid used, there is an increasingly clear concern to identify the real indications of fluid replacement in the perioperative period.
One of the most prevalent strategies is Goal-Driven Therapy (GOT), which has been repeatedly used to significantly improve the results of hemodynamic optimization, both in the short and long term, [19]T[20]he TGO approach is focused on maximizing (optimizing) cardiac output (CO) by incremental fluid administration[21].
The National consensus conducted in 2016 concluded that the success in Goal-Guided Therapy (GOT) and other restrictive fluid strategies suggest that perioperative fluid planning should emphasize that fluid therapy is administered only when there is a clea[22]r indication using the inclusion criteria presented in table 01.
Furthermore, regarding the targeted therapeutic strategy, there are 9 studies in which a therapeutic strategy directed to a goal was also used to maximize hemodynamic variables related to the flow of surgical, intra and postoperative patients[23]. The studies found that the treatment strategy reduced gastrointestinal complications and length of hospital[24] stay, both in the ward and in intensive care units[25].
Concomitantly with the initiation of therapy, it is important to emphasize that, once initiated, the evaluation of responsiveness before fluid administration can not only help in the detection of patients in need of fluids, but also avoid unnecessary and harmful fluid overload. This evaluation consists, for example, in observing functional dynamic parameters such as changes in cardiac output during preload-modifying maneuvers – such as passive elevation of the lower limbs[20].
In high-risk surgical patients, the use of a hemodynamic protocol to maintain tissue perfusion decreased postoperative organ mortality and failure[26]. Monitoring cardiac output by calculating oxygen transport and consumption also helped guide therapy[18].
4. FINAL CONSIDERATIONS
The ideal replacement regimen would be one that decreased mortality and improved quality of life, decreased multiple organ failure and blood use, and was low-cost. This scheme has obviously not yet been achieved. As there is no evidence-based guideline, in practice a combination of crystalloids and colloids is used, and it is more important to know how to replace than to know what to replace.
Generally, fluids should be applied like any other medication, respecting their individual indications and limitations. Referring to this, advantageous aspects of perioperative volume replacement with iso-oncotic solutions are understandable, although additional randomized controlled trials are needed to prove its relevance to the outcome. Summarizing the arguments cited above, a rational fluid administration strategy could be to treat patients undergoing low-risk surgery with negligible intravascular volume loss with crystalloid infusion and use a combination of crystalloid and colloid administration, carefully titrated based on hemodynamic measurements.
Finally, we conclude that goal-guided therapy in the perioperative period is associated with reductions in complications and duration of hospitalization. The beneficial effects of OT can be achieved, thus avoiding the difficulties of admission to the Intensive Care Units.
5. REFERENCES
BRANDSTRUP, B.; SVENDSEN, P. E.; RASMUSSEN, M.; et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? British Journal of Anaesthesia. United Kingdom, v. 109, n.2 , p. 191–199, 17 jun. 2012. Disponível em: <https://ac-els-cdn.ez3.periodicos.capes.gov.br/S000709121732888X/1-s2.0-S000709121732888X-main.pdf?_tid=bde7ae63-beb3-46c9-9adc-e0f04482d0cf&acdnat=1539636094_9a27cfa1829cc5679acd68973a0e7357>. Acesso em: 15 out. 2019.
BUNDGAARD-NIELSEN, M.; HOLTE, K.; SECHER, N. H.; KEHLET, H. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiologica Scandinavica. USA v. 51, n. 3, p. 331–340, mar. 2007. Wiley/Blackwell (10.1111). Disponível em: <http://doi.wiley.com/10.1111/j.1399-6576.2006.01221.x>. Acesso em: 16 out. 2019.
GOW, K. W.; PHANG, P. T.; TEBBUTT-SPEIRS, S. M.; et al. Effect of crystalloid administration on oxygen extraction in endotoxemic pigs. Journal of Applied Physiology. USA, v. 85, n. 5, p. 1667–1675, nov. 1998. American Physiological SocietyBethesda, MD . Disponível em: <http://www.physiology.org/doi/10.1152/jappl.1998.85.5.1667>. Acesso em: 15 out. 2019.
GROCOTT, M. P. W.; MYTHEN, M. G.; GAN, T. J. Perioperative Fluid Management and Clinical Outcomes in Adults. Anesthesia & Analgesia. USA, v. 100, n. 4, p. 1093–1106, 2005. Disponível em: <https://insights.ovid.com/crossref?an=00000539-200504000-00032>. Acesso em: 15 out. 2019..
GURGEL, S. T.; DO NASCIMENTO, P. Maintaining Tissue Perfusion in High-Risk Surgical Patients. Anesthesia & Analgesia. USA, v. 112, n. 6, p. 1384–1391, 2011. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/21156979>. Acesso em: 16 out. 2019..
HAMILTON, M. A.; CECCONI, M.; RHODES, A. A Systematic Review and Meta-Analysis on the Use of Preemptive Hemodynamic Intervention to Improve Postoperative Outcomes in Moderate and High-Risk Surgical Patients. Anesthesia & Analgesia. USA, v. 112, n. 6, p. 1392–1402, 2011. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/20966436>. Acesso em: 16 out. 2019.
HOLTE, K.; SHARROCK, N. E.; KEHLET, H. Pathophysiology and clinical implications of perioperative fluid excess. British Journal of Anaesthesia. United Kingdom, v. 84, n. 4, p. 622-632, oct. 2002. Disponível em: <https://www.ncbi.nlm.nih.gov/pubmed/12393365>. Acesso em: 16 out. 2019.
KELLUM, J. A. Saline-induced hyperchloremic metabolic acidosis. Critical care medicine. New York, v. 30, n. 1, p. 259–61, 2002. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/11902280>. Acesso em: 15 out. 2019.
LEE, T. H.; MARCANTONIO, E. R.; MANGIONE, C. M.; et al. Derivation and Prospective Validation of a Simple Index for Prediction of Cardiac Risk of Major Noncardiac Surgery. Circulation. Dallas, v. 100, n. 10, sep. 1999. Disponível em: < https://www.ncbi.nlm.nih.gov/pubmed/10477528>. Acesso em: 15 out. 2019.
LORENTZ, M. N. Reposição volêmica perioperatória. Rev Med Minas Gerais. Minas Gerais, v. 20, n. 4, p. 47–56, 2018. Disponível em: <http://rmmg.org/artigo/detalhes/1025>. Acesso em: 15 out. 2019.
MANGANO, D. T.; LAYUG, E. L.; WALLACE, A.; TATEO, I. Effect of Atenolol on Mortality and Cardiovascular Morbidity after Noncardiac Surgery. New England Journal of Medicine. Canada, v. 335, n. 23, p. 1713–1721, 1996. Massachusetts Medical Society . Disponível em: <http://www.nejm.org/doi/abs/10.1056/NEJM199612053352301>. Acesso em: 15 out. 2019.
MORETTI, E. W.; ROBERTSON, K. M.; EL-MOALEM, H.; GAN, T. J. Intraoperative Colloid Administration Reduces Postoperative Nausea and Vomiting and Improves Postoperative Outcomes Compared with Crystalloid Administration. Anesthesia & Analgesia. USA, v. 96, n. 2, p. 611–617, 2003. Disponível em: <https://insights.ovid.com/crossref?an=00000539-200302000-00056>. Acesso em: 15 out. 2019.
MYTHEN, M. G.; WEBB, A. R. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Archives of surgery. Chicago, v. 130, n. 4, p. 423–9, 1995. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/7535996>. Acesso em: 16 out. 2019.
ØSTGAARD, G.; REED, R. K. Interstitial fluid accumulation does not influence oxygen uptake in the rabbit small intestine. Acta Anaesthesiologica Scandinavica. USA, v. 39, n. 2, p. 167–173, 1995. Wiley/Blackwell (10.1111). Disponível em: <http://doi.wiley.com/10.1111/j.1399-6576.1995.tb04037.x>. Acesso em: 15 out. 2019.
PEARSE, R.; DAWSON, D.; FAWCETT, J.; et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Critical Care. New York, v. 9, n. 6, p. R687, 2005. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/16356219>. Acesso em: 16 out. 2019.
PEREL, A.; HABICHER, M.; SANDER, M. Bench-to-bedside review: Functional hemodynamics during surgery – should it be used for all high-risk cases? Critical Care. New York, v. 17, n. 1, p. 203, 2013. BioMed Central. Disponível em: <http://ccforum.biomedcentral.com/articles/10.1186/cc11448>. Acesso em: 15 out. 2019.
POLDERMANS, D.; BOERSMA, E.; BAX, J. J.; et al. The Effect of Bisoprolol on Perioperative Mortality and Myocardial Infarction in High-Risk Patients Undergoing Vascular Surgery. New England Journal of Medicine. Canada, v. 341, n. 24, p. 1789–1794, 1999. Massachusetts Medical Society. Disponível em: <http://www.nejm.org/doi/abs/10.1056/NEJM199912093412402>. Acesso em: 15 out. 2019.
RAOBAIKADY, R.; DINESH, S.; HACKING, M.; WIGMORE, T. Cardiopulmonary exercise testing as a screening test for perioperative management of major cancer surgery: a pilot study. Critical Care. New York, v. 11, n. Suppl 2, p. 250, 2007. BioMed Central. Disponível em: <http://ccforum.biomedcentral.com/articles/10.1186/cc5410>. Acesso em: 15 out. 2019.
RIELLA MC, RIELLA CV, PACHALY MA, RIELLA LV. Metabolismo da água. In: Riella MC, org. Princípios de nefrologia e distúrbios hidroeletrolíticos. 5ª ed. Rio de Janeiro, Brasil: Guanabara Koogan; 2010. p. 105-38.
RUTTMANN, T. G.; JAMES, M. F.; VILJOEN, J. F. Haemodilution induces a hypercoagulable state. British Journal of Anaesthesia. United Kingdom, v. 76, n. 3, p. 412–414, 1996. Oxford University Press. Disponível em: <http://linkinghub.elsevier.com/retrieve/pii/S0007091217435483>. Acesso em: 16 out. 2019.
SHOEMAKER, W. C.; APPEL, P. L.; KRAM, H. B.; WAXMAN, K.; LEE, T. S. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. Glenview, v. 94, n. 6, p. 1176–86, 1988. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/3191758>. Acesso em: 16 out. 2019.
SILVA, E. D.; PERRINO, A. C.; TERUYA, A.; et al. Brazilian Consensus on perioperative hemodynamic therapy goal guided in patients undergoing noncardiac surgery: fluid management strategy – produced by the São Paulo State Society of Anesthesiology (Sociedade de Anestesiologia do Estado de São Paulo – SAESP). Brazilian Journal of Anesthesiology (English Edition). São Paulo v. 66, n. 6, p. 557–571, 2016. Sociedade Brasileira de Anestesiologia. Disponível em: <https://linkinghub.elsevier.com/retrieve/pii/S0104001416301750>. Acesso em: 15 out. 2019.
SINCLAIR, S.; JAMES, S.; SINGER, M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ (Clinical research ed.). London, v. 315, n. 7113, p. 909–12, 1997. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/9361539>. Acesso em: 16 out. 2019.
SKYTTE LARSSON, J.; BRAGADOTTIR, G.; KRUMBHOLZ, V.; et al. Effects of acute plasma volume expansion on renal perfusion, filtration, and oxygenation after cardiac surgery: a randomized study on crystalloid vs colloid. British Journal of Anaesthesia. United Kingdom, v. 115, n. 5, p. 736–742, 2015. Oxford University Press. Disponível em: <https://linkinghub.elsevier.com/retrieve/pii/S0007091217310759>. Acesso em: 15 out. 2019.
SUBRAMANIAM, B.; SUBRAMANIAM, K.; PARK, K. W. Volume Replacement Strategies and Outcome. International Anesthesiology Clinics. USA, v. 48, n. 1, p. 115–125, 2010. Disponível em: <https://insights.ovid.com/crossref?an=00004311-201004810-00009>. Acesso em: 16 out. 2019.
TOCANTINS, L. M.; CARROLL, R. T.; HOLBURN, R. H. The Clot Accelerating Effect of Dilution on Blood and Plasma. Relation to the Mechanism of Coagulation of Normal and Hemophilic Blood. Blood. Washington, v. 6, n. 8, 1951. Disponível em: <http://www.bloodjournal.org/content/6/8/720.short?sso-checked=true>. Acesso em: 15 out. 2019.
TOTE, S. P.; GROUNDS, R. M. Performing perioperative optimization of the high-risk surgical patient. British Journal of Anaesthesia. United Kingdom, v. 97, n. 1, p. 4–11, 2006. Oxford University Press. Disponível em: <http://linkinghub.elsevier.com/retrieve/pii/S0007091217351772>. Acesso em: 15 out. 2019.
APPENDIX – REFERENCES IN FOOTNOTE
2. Hamilton, 2011.
3. Holte, 2002.
4. Grocott, 2005.
5. Brandstrup, 2012.
6. Lorentz, 2018.
7. Subramaniam, 2010.
8. Riella, 2010.
9. Lee, 1999.
10. Raobaikady, 2007.
11. Tote, 2006.
12. Skytte Larsson, 2015.
13. Moretti, 2003.
14. ØStgaard, 1995.
15. Gow, 1998.
16. Kellum, John.
17. Ruttmann, 1996.
18. Tocantins et al. 1951
19. Gurgel; Nascimento, 2011.
20. Hamilton, Cecconi, Rhodes, 2011.
21. Perel, Habicher, Sander, 2003.
22. Silva, Et al. 2016.
23. Bundgaard-Nielsen, 2007.
24. Mythen, 1995.
25. Sinclair, Stephen. James, Sally. Singer, Merrill, 1997.
26. Pearse, Ruppert, 2005.
[1] Medical. Postgraduate in Intensive Care Medicine for Adults.
Sent: December, 2019.
Approved: January, 2020.















