BARRETO, Genesson dos Santos [1]
BARRETO, Genesson dos Santos. Blood transfusion: The patient/Donor Case Cuiabá-MT. Multidisciplinary Core scientific journal of knowledge. Year 1. Vol. 8. pp. 276-314. September 2016. ISSN. 2448-0959
SUMMARY
Purpose of this study is to analyze all the blood transfusion in all its stages. Addressing since the preceding procedures the donation, through the analysis of the material, separation and selection, choice of receivers until the transfusion. In this way, search show, raise awareness and raise awareness about the great importance of the subject.
Keywords: blood transfusion, blood products, Blood Transfusion Reactions.
Introduction
The first blood transfusion was performed on animals in the 17th century by Richard Lowoer, Oxford. Two years later, Jean-Baptiste Denis, doctor and professor of mathematics, held a blood infusion in a mental patient. At that time, the transfusions were heterólogas and defended with the argument that the animal blood was less contaminated of vices and passions. (GINGERICH, 1986).
In 1788, Pontick and Landois, after several failed attempts, obtained positive results performing homologous transfusions (between animals of the same species), concluding that could be beneficial. In 1818, James Blundell performed the first blood transfusion in women with postpartum hemorrhages. (SCHMOTZER et al., 1985)
At the end of the 19th century, scientists are still researching with transfusion reactions and problems with blood clotting. The first transfusion preceded the completion of compatibility tests was conducted in 1907, by Reuben Ottenber. (GINGERICH, 1986)
In 1914, Hustin employed sodium citrate and dextrose as anticoagulant solution and diluent for transfusions and, the following year, Lewisohn has determined the minimum quantity for anticoagulation. In 1936, the first blood bank emerged in Barcelona during the Spanish Civil War. (SCHOMOTZER et al., 1985)
In the 20th century, the progress of blood transfusions was signed through the discovery of the ABO system and the identification of the Rh factor, the scientific job of anticoagulants, improved collection and infusion and the contraindications. (HOSGOOD, 1990)
In Brazil, in the 40, comes the STS (Blood Transfusion Service) to develop scientific and social activities. At the end of this decade, is promoted the I Paulista Congress of Haemotherapy, which provided the basis for the Foundation of the Brazilian Society of Haematology and haemotherapy. In 1965, the Ministry of health creates the national Hemotherapy to establish standards for the donation and receipt of the blood. (JUNQUEIRA, 2005)
In the years 80, the creation of the National blood Policy, the selfless donation of blood of the SBHH and the 1988 Constitution gave another dimension to the brazilian setting standards to organize Hematology blood donation, now controlled and funded by the State. (JUNQUEIRA, 2005)
Currently, the blood transfusion is one of the five most procedures performed in the world and your prescription is made for 10% of all hospitalized patients. (Proceedings from the National Summit on Overuse, 2013)
According to the Ministry of health, around 3,500,000 of units of blood are collected annually in Brazil. Soon, the biggest challenge is to increase the donation, because donors represent only 1.7% of the brazilian population. (LUDWIG, 2005).
Still, the blood transfusions are modernized and their methodologies more insightful. The common problems in the past on the issue of lack of knowledge on systems ABO, tranfusionais contamination and conservation of blood brought problems that are currently handled by the modernization of the process. Sorting and separation of blood products, today, in Brazil with a very advanced technological level and this is balanced with the demand problem: lack of blood donors. In addition to this difficulty, epidemiological issue of diseases like zika and religious social conflicts affect from the transfusion of blood collection due hemocomponents.
Therefore, this work aims to address since the preconditions for the donation, the processing of blood donated to the infusion in the patient. In addition, knowledge about the use of blood products in blood transfusion.
2. Prerequisites
Although the basic requirements for donating blood to hemocentro hemocentro due vary from your requirements and regulations, there are a number of conditions for a blood donation is efficient and does not cause harm to both the giver and the receiver. The Fundação Pró-Sangue-blood bank of São Paulo is a reference in Latin America and has great credibility in their actions. As a result, in this present study, we used the "basic requirements for the donation" on the website of the Foundation. Such requirements are classified in: Basic, temporary and definitive. The basic ones are: be in good health; have between 16 and 69 years, since the first donation was made to the 60 years; weigh at least 50 kg; be rested; be fed (avoid greasy food in 4 hours priors to donation); submit original documents issued by official organ. How to: Temporary cold; body temperature; medicines whose suspension is greater than 48 hours; pregnancy; 90 days after vaginal delivery and 180 days after caesarean section; breastfeeding; ingestion of alcoholic beverages in 12 hours background the donation; Tattoo and permanent makeup; situations in which there is greater risk of acquiring sexually transmitted diseases; have been in States where there is higher prevalence of Malaria in the last 12 months (AC, AM, RO and MT) and any endoscopic examination (e.g., colonoscopy). And finally the Definitive Impediments are based on: testing positive for HIV; hepatitis testing after the 10 years of age; having already presented malaria; have chagas ' disease; have presented some type of cancer; blood clotting problems; use of insulin; have elephantiasis; is subjected to organ transplants and have leprosy. (PRÓ-SANGUE, 2016).
3. Donation steps
After meet the prerequisites, the donor is transferred to the "Steps of Donation" that determine whether the individual is in good health, free from diseases that can be transmitted by donated blood and if he is able to tolerate the procedure without complications (HEMATOLOGY TECHNICIAN, 2013). Such steps include: Registration, Anemia, vital signs and weight, sorting, Auto Delete Vote, collection, Snack. The Record is where the patient reports on your data both for blood donation record as to possible future donations. In addition, the donor generally receives an identification number. The Anemia test evaluates the candidate's haemoglobin level usually by fingerstick, collecting so the hematocrit (Ht) or the concentration of hemoglobin (Hb) whose values are Hb: 12.5 g/dl or Ht: 38%. In a sequence will be assessed the vitals, heart rate, blood pressure, and finally the weight. It is during the Screening clinic that the candidate responds to a private and confidential interview with the purpose of evaluating risks for him or for the receiver. The deletion Vote in which the patient can make an option so that your blood is donated. According to Eugenia Maria (technical on HEMOTHERAPY, 2013) is a mechanism of questionable effectiveness, usually used by donors to evaluate have omitted important facts in the interview that could compromise the safety of donated blood. The next step is the collection itself, in which using a sterile disposable needle is collected 450 mL of blood for tests required. And, lastly, it is offered a snack should be consumed in the cafeteria of the collection.
4. Care after donation
Is established by the ANVISA and the Ministry of health that the "pós-doação Care" is precisely aimed at the health of the donor in order to ensure its integrity even if there is no transfusion risk-free. Such care are based on relax and remain seated in order to avoid dizziness and lightheadedness; together is given a snack for the donor to raise your blood glucose and to the replacement of the net loss. (MS ORDINANCE n° 1,353).
5. Strategies for the reduction of blood transfusions
It is contemplated that the technical responsibility for the services of haemotherapy, preferably Hematologist/Haemoteraphists, which will have among its responsibilities the determination of adequacy of blood transfusion indications and components within their institution (MANUAL of RATIONAL USE of BLOOD, 2011). In addition, it is recommended by who the questioning the need of elective transfusions and consider using strategies and drugs that can reduce or eliminate the use of chemicals. These "strategies for the reduction of blood transfusions" consist from the rationalisation of hospital equipment as the "lines" arteries to using medicines with different purposes for anemia and active coagulation factors. Azambuja (apud ALTERNATIVE METHODS to BLOOD TRANSFUSION, 2013) the ethical, moral and legal transformations are in scientific and technical progress of recent years which brought new practices in health situations, modifying the professional relationship with the client in order to guide the duties and moral obligations and more respect for autonomy. One of these strategies has:
- Reduce blood samples for laboratory tests
Analyze the need of the blood request, prioritizing the use of medicines that require the least amount of blood samples in the course of treatment (ex: Heparin-an anticoagulant).
- Rationalize the use of arterial lines
Analyze judiciously the need of using any arterial lines (thin catheters inserted into arteries to blood gas).
- Optimize patient care with acute bleeding
Shorten the time between the patient's intake and its surgical treatment, in addition to using preferably crystalloids as saline to volume expansion (prevents the vessel colabação due to hemorrhage).
5.1 use of Hemostatic agents and anfibrinolíticos
The use of Desmopressin: analogue of the synthetic vasopressin. Its mechanism of action increases levels of Von Willebrand factor and factor VII, encouraging homeostasis. It shortens the time of bleeding in patients with platelet dysfunction associated with the use of anti-inflammatory drugs-estereoidais.
Your directions are in cases of complex heart surgery and hemophilia type A (hemophilia B has no indication, because it has no effect on the factor IX).
5.2. Use of erythropoietin, iron and vitamin B12
The hormone erythropoietin is produced by the kidney and is the main regulator of the production of red blood cells. Can be useful in some types of anemia. The use of iron is also indicated for the treatment of anemia and vitamin B12 can cause a type of anemia as it participates in cell divisions of the nucleated erythrocyte stages.
Although undergoing studies, according to the GLOBE website, a "synthetic" blood may be tested in 2017. A blood made of stem cells obtained from umbilical cord. "The proposal is that this blood to provide specialized treatment for specific groups of patients" (NICK WATIKINS-NHS, 2016). There will be tests for the issue of adverse transfusion reactions. (The GLOBE, 2016)
6. Request of donated blood
All blood transfusion or components must be prescribed by a doctor, and must be recorded in the medical record of the patient (ORDINANCE No. 1353 of 6/13/11). The University Hospital Julius Muller of Cuiabá follows these standards also require that the form must bear the Hemocomponente requested, as well as the laboratory results that justify the indication of such. In accordance with the applicable law Art. 106, the modalities of transfusion are: programmed (given time); of routine (if achieve within 12:00 am); of urgency (if performing one of 3:00) and emergency when the delay of transfusion can cause hazard to the patient's life. (TRANFUSIONAL PRIMER-HUMG, 2016). The requesting doctor fills a form APAC – high-complexity Procedures permit-to forward to the blood bank. In emergency cases, this form is not filled, however certain requirements are still present as a term of responsibility of both the doctor and the patient and pre-transfusion (ABO compatibility) must be submitted (TRANFUSIONAL-HUJM AGENCY, 2003).
7. Blood donation and the Zika Virus
Currently, have been too much in the Zika virus and its complications. Although studies about are being developed, a patient has been infected by the virus through blood transfusion in December of last year (the Globe, 2015). The circulation of the virus was confirmed through a test called PCR high cell biotechnology. In this present year of 2016, France has tightened blood donation because of the virus. The patient from an infected region cannot donate blood within a period of 28 days. However, further studies are needed to evaluate the real effectiveness, setting the seroprevalence of their transmission through transfusion of blood products and determine the ability to generate a reaction in the receiver (TECHNICAL NOTE – ANVISA, 2015). The same note is informed that candidates for donation of blood have been infected by the virus Zika in Brazil, after clinical and laboratory diagnosis, should be considered unfit for a period of 30 days after full recovery.
8. The issue of Jehovah's witnesses
Another standoff on the issue of blood transfusion is related to the Jehovah's witnesses. Even if label that religion does not use blood and there is still a strong religious ethnocentrism in the country, there is a certain relation of flexibility between perspectives as well. The Federal Council of Medicine establishes a certain conduct to the doctor in the face of refusals on blood transfusion, however it consists of respect for the will of the patient provided that there is no imminent risk of life. Even shy, many medical communities recognize the right of the patient to reject certain medical treatments (LEIRIA, 2004). Have the patient a Jehovah's witness, in small part, using methods "without blood" present today. The interpretation comes changed over time with the emergence of new techniques in medicine. The fact is that many doctors are unaware that the Jehovah's witnesses tolerate various treatments involving blood; the blood products (white blood cells, red blood cells, plasma and platelets) stored from other individuals are not accepted; as for the blood products (small fractions) are considered as they are not considered blood (soul) on doctrine (AZAMBUJA, 2010). The question though, is relaxed in mutual agreement. Moreover, in the face of modernity the biblical interpretations are varied, and often the patient carries their own convictions and establishes himself religious restrictions.
7. ABO blood system
In the first half of the 20th century, the Austrian researcher Karl Landsteiner (Nobel Laureate in 1930) found differences in the blood of humans-clarifying the deaths that occurred after blood transfusions. The mismatch before applicant, between donor and receiver if the immune reaction between the substances present in the blood plasma and the red blood cells. This reaction, in turn, is characterized as agglutination (clumping) of red blood cells-that will block, therefore, the capillaries. The portions of blood suffered clumps from certain antigens, in red blood cells, known as aglutinogênios (A and B) while aglutinadoras plasma substances have been called agglutinins (anti-a and anti-b). (Institute of HEMATOLOGY, 2015)
The determinant antigens (subsequent glycosphingolipid buildup), in this case, Terminal residues are found in carbohydrates that exist in cells and in the secretions. These are synthesized by specific enzymes, which catalyze the reactions of transglicolização, and are at the ABO locus (long arm of chromosome 9). (BATISSOCO & NOVARETTI, 2003)
Multiple alleles (three genes located on the long arm of chromosome 9, as has been said above) determine the blood type in humans. They are: IA, IB and i. Blood genotype is given by a pair of these alleles. Being IA and IB dominant in relation to (i), but not showing dominance amongst themselves. The individual receives a paternal gene and another. In this way, a hypothetical couple in which the parent has the phenotype of blood type O and his mother has blood type B phenotype cannot have children with the phenotype of type A, because there's no gene IA – that needs to be present at par or accompanied by (i) an individual with this character. (BATISSOCO & NOVARETTI, 2003)
| Genotype | Phenotype |
| The | IAIA or IAi |
| (B) | IBIB or IBi |
| AB | IAIB |
| The | II |
Table 9.1: relationship between the genotypes and phenotypes of the ABO System blood.
9.1. Aglutinogênios
The aglutinogênios (A and B) correspond to antigens (substances capable of initiating an immune response) found on the surface of red blood cells, resulting in blood phenotype of an individual. (JUNQUEIRA & CARNEIRO, 2008)
9.2 Agglutinins
The cold agglutinins (anti-a and anti-b) correspond to IgM class antibodies (proteins used by the immune system to identify the antigens present in foreign bodies and thus neutralize them) found in the blood plasma. (JUNQUEIRA & CARNEIRO, 2008)
9.3. Blood types
The knowledge of the patient's blood type is, in many cases, extremely important information for healthcare professionals-because it is inherent to the success of a blood transfusion. (COLSAN-ASSOCIAÇÃO BENEFICENTE BLOOD COLLECTION, 2013)
Blood type a: can donate red cell components for individuals with the same type and for humans with type AB; and receive, in addition, donors can receive blood products of individuals of type (HOFFBRAND & MOSS, 2013) the individual is bearer of the genotype IAIA or IAi genotype. (COLSAN-ASSOCIAÇÃO BENEFICENTE BLOOD COLLECTION, 2013)
Blood type b: can donate cells components for individuals with the same type or type AB; and receive from donors of type B or o. (HOFFBRAND & MOSS, 2013) in this case, is carrying the IBIB or IBi genotype, genotype. (COLSAN-ASSOCIAÇÃO BENEFICENTE BLOOD COLLECTION, 2013)
Blood type AB (universal receiver): can donate red cell components for individuals with the same type; and receive, in addition to donors of type AB, of individuals of type A, B and o. (HOFFBRAND & MOSS, 2013). Has the IaIb genotype. (COLSAN-ASSOCIAÇÃO BENEFICENTE BLOOD COLLECTION, 2013)
Type O blood (universal donor): can donate red cell components for individuals from all the blood typings; and receive, only, of the type also donors. (HOFFBRAND & MOSS, 2013) it is noteworthy that has blood genotype ii. (COLSAN-ASSOCIAÇÃO BENEFICENTE BLOOD COLLECTION, 2013)
| Blood type | Genotype | Aglutinogênios in red cells | Cold agglutinins in plasma |
| The | IAIA or IAi | The | Anti-B |
| (B) | IBIB or IBi | (B) | Anti- |
| AB | IAIB | And (B) | – |
| The | II | – | Anti-A and Anti-B |
Table 9.2: relationship between phenotypic and genotypic characters with the aglutinogênios and present agglutinins in the blood.
Because of the presence of agglutinins (antibodies) in the blood plasma plasma donations configuration and components containing plasma do not get how when it comes to red cell components (elucidated above). AB plasma (without antibodies) can be given to any ABO group. The plasma (with anti-b) can be administered to patients or the plasma B (with anti-a) can be administered to patients B or. Lastly, the plasma (with anti-a and anti-b) can only be administered to patients (who, 2013)
In the case of crioprecepitados, the blood received must be of the same patient's blood type in ABO typing. (Who .2013)
9.4. Bombay effect-False 0
In the city of Bombay (Mumbai), located in India, there is a high incidence of a genetic modification known as Bombay or false Effect. The share of less than 1% of the population is identified as if they were blood, even being homozygous or heterozygous type A and B, or AB-that when used traditional techniques of determining the blood groups. (VIEIRA, 2013)
This phenomenon occurs because, a dominant allele H determines the production of a factor responsible for the phenotype of the ABO system. Therefore, individuals HH or Hh synthesize an enzyme that is responsible for formation of the H Antigen, which is transformed into A or B antigen – inherent to determine the blood groups A, B and AB, in traditional tests. When this allele is not present, i.e. in cases of homozygous recessive individuals (hh), is manifested a phenotype of type O blood regardless of its genotype true, since it is synthesized an enzyme inactive. On these facts, to detect if a person is really the or a fake, it is necessary a test in which it is applied the antibody h in a drop of blood. When there are clumping of this sample, the individual has the genotype for the blood; If not, is a fake, it is not possible to make the detection of your actual blood group. (VIEIRA, 2013)
10. HR System
Landsteiner (descortinador of the ABO system), together with Wiener, discovered a second system of blood groups important to the blood transfusion. These researchers found a membrane antigen, HR or Rhesus (species name of monkeys used initially in the experiment), which stimulates the production of antibodies, called RH. (HOFFBRAND & MOSS, 2013)
When mixing the blood of several humans with serum containing RH, about 85% of samples surveyed suffered by agglutination Antigen Rh and were called Rh +. The other 15% that didn't suffer this agglomeration of erythrocytes were called Rh-. (HOFFBRAND & MOSS, 2013)
This is a multiple allele inheritance, in which two pairs of genes are involved. However, it is considered the participation of only one of these pairs, the RhD, in the production of the Rh factor-therefore, can be considered a case of simple Mendelian Inheritance. Gene D, dominant, determines the presence of the Rh factor, while the recessive gene (d), makes the absence of that factor. Alternative RNA splicing of the RhCE gene generate two proteins that code C, c, E or E. But it is common to use a simplified nomenclature for the Rh phenotype. (HOFFBRAND & MOSS, 2013)
Therefore, a patient receiving blood Rh-Rh + blood agglutination will not suffer, because the Rh-blood carrier does not have the RH antibody. But after this first contact, the patient starts the production of RH antibodies and your system may trigger a process of agglutination of the red blood cells too dangerous and even fatal. (HOFFBRAND & MOSS, 2013)
Having in mind these concepts, we conclude that the blood-appears as a universal donor. Although, the blood type AB + appears as the universal receiver. Remembering that this is valid when it is not a question of blood transfusions or plasma components with the presence of plasma. (HOFFBRAND & MOSS, 2013).
10. Processing of blood Donated
After collecting donated blood will be held imunoematológicos and serological tests, as well as its processing in hemocomponents (fractional parts of whole blood). These techniques are performed in order to ensure the safety of the receiver, increasing the longevity of blood components and enhance the use of the various blood products. (Guide to using blood products-Ministry of health, 2008)
11.1. Serological tests
After collection, blood is separated about 10 ml to be performed some tests in order to detect diseases that can be transmitted through transfusion.
In Brazil the mandatory analyses today are HIV, hepatitis B and C, HTLV I and II, Syphilis and Chagas disease. In endemic regions of Brazil can still be done the test for diagnosis of malaria.
Human immunodeficiency virus (HIV): tests in order to detect HIV antibodies, which become detectable approximately 21 days after exposure to infection. In the immunological window period in which viremia, but not if the antibodies test identifies presents negative result, which can cause the blood to be donated is contaminated. So it is important that a careful selection of candidates for donation. (Clinical use of Blood-who)
HTLV-i and II: serological tests are made specific for antibodies anti-HTLV-I/II in the blood. As most of the carriers are asymptomatic, must pay attention if the forms of transmission, which are similar to HIV, to select candidates for donation.
Hepatitis B and C: all donated blood should be tested for HBsAg, which pair is the surface antigen of the hepatitis B virus. Individuals who regularly receive blood products should be vaccinated for hepatitis B, since persons infected with hepatitis C anti-HCV antibodies identified.
Syphilis: to prevent transmission by blood transfusion carried out tests for serological evidence of infection by Treponema pallidum, bacteria responsible for the infection.
Chagas disease: there are several tests that can diagnose the disease, however the RDC 343 recommends the establishment of a imunoenzimatico high test sensitivity and specificity. (Technical Manual for investigation of the transmission of diseases by blood – ANVISA, 2004)
11.2. Separation of blood products
The fractionation of blood donated is a process that requires specific care on your action. In Brazil it follows the resolution of the Collegiate Board of national health surveillance Agency RDC n° 24, 1/24/2002, being used the refrigerated centrifuge method which reduces potential contamination and microbial arms proliferation. Due to difference in density and size of molecules, this process divides the blood in three phases, the concentrate of red blood cells at the bottom, the leucoplaquetária layer (buffy coat) and plasma. (Guide to using blood products-Ministry of health, 2008). Each fraction are added anticoagulants solutions and preserving additive solutions to increase the durability of components and prevent clotting. The storage occurs in refrigerators with average temperature of 4ºc. (Clinical Use of blood-who)
11.2.1. Red cell concentrate (CH)
Obtained through the whole blood by centrifugation refrigerated. Consists primarily of red cells, with small amount of plasma. In a bag of whole blood with 450 ml, remove approximately 220 to 340 ml of CH. After being processed can last on 21 storage days to 42 days depending on the substances added to prolong its shelf life. If it is added only anticoagulants preserving substances (ACD, CPDA-1 or CPD) the validity of the CH is smaller, but if it's additive solutions, such as SAG added-M (composed of saline, adenine, glucose and mannitol) increases storage period. (Manual of Haemotherapy, 2011).
11.2.2. Platelet concentrate (PC)
Can be obtained through the donation of whole blood or by apheresis. In obtaining for whole blood there are two ways of obtaining. The first is by means of centrifugation of the blood in two subsequent steps, getting Rico plasma in platelets, and later the CP. The second through leucoplaquetária layer extraction (buffy coat) with use of a sterile bag top and botton type specific, in which plasma supernatant is removed by higher output (top) and the lower exit CH (botton). The leucoplaquetária layer remains in original bag and can be grouped with other bags of same content being made subsequent sedimentation and centrifugation to separate and transfer platelets to a satellite scholarship. This latter method allows reduction in leukocyte content of up to 90% which can reduce the incidence of febrile reactions in the receiver. (Guide to using blood products-Ministry of health, 2008).
In obtaining by Apheresis the donor is connected to a specialized system in which blood is withdrawn and by a mechanical process, on the same machine, the CP is separated and stored in sterile pouch. The remainder of the blood that is not used is reinfused into the donor, which prevents anemia frames, since red blood cells come back to the body. Another advantage of this process is the amount of platelets, which can be up to seven times greater when compared to obtaining via whole blood. (Clinical Use of blood-who).
11.2.3. Plasma
Plasma can be obtained several derivations depending on how was separated.
The FFP (PFC) is obtained by centrifugation of whole blood and directed to a satellite, pouch or can still be obtained by apheresis. Is the blood made up of acellular water, proteins (albumin, globulin and clotting factors), carbohydrates and lipids. (Guide to using blood products-Ministry of health, 2008). Has level considered coagulation factor VII, about 70% when compared to fresh frozen plasma. (Clinical Use of blood-who). For preservation purposes is frozen 8 hours after your collection and kept frozen at temperatures below 18° C negative. If frozen between temperatures of -25° C -18° C has validity of 12 months at temperatures below -25° C validity increases to 24 months. The freezing is done to preserve coagulation factors, fibrinolysis, albumins, immunoglobulins and other proteins and minerals. For its use in patients is unfrozen and may be stored for 24 hours at the temperature from 1 to 6th C. (Guide to using blood products-Ministry of health, 2008).
The plasma free of Cryoprecipitate (PIC) is obtained with the removal of Cryoprecipitate in closed system. If the approximate volume collected is 150 to 200 ml. Storage and expiration are similar to PFC. The PIC is depleted of clotting factor VIII, Fibrinogen and high molecular weight multímeros of Von Willebrand factor, although metal responsible for their metabolism.
There are still 24-hour plasma (P24) acquired by centrifugation of whole blood between eight and 24 hours after blood collected and frozen within 1 hour to 30ºc. After this freezing process immediately is stored similarly to PFC with the same validity. Presents some variables decreases clotting factors, such as factor V and VIII. (Guide to using blood products-Ministry of health, 2008).
11.2.4. Cryoprecipitate (CRYO)
Is a concentrate of some insoluble proteins at low temperatures (1 C to 6° C) plasma-derived. Obtained from the thawing of a PFC, capturing the supernatant plasma and leaving the original rash protein pouch and small amount of plasma, about 10 ml. The material of the bag is frozen within 1 hour and is valid for a period of one year. Consists of high molecular weight glycoproteins such as factor VIII (80 to 150 units), von Willebrand factor (100 to 150 units), Fibrinogen (150 to 250 mg), factor XIII (50 to 75 units) and fibronectin. (Guide to using blood products-Ministry of health, 2008).
12. Clinical indications of the main blood products
Modern transfusion therapy guides fundamentally the use of specific blood components that are indicated to treat particular medical condition of the patient (RAZOUK et al., 2004). The advantages of this guidance will be obtained as a matter of fact when there is a real need for transfusion therapy and prescription with the clinical indication, taking into account the risks, benefits and alternatives of this method (MAKKAR et al., 2013). To this end, requests are necessary, particulars, routine after the start of transfusion and specific material. In addition, of course, of pré-transfusionais tests. These processes is defined as:
- Requirements: must be made a proper request, describing the urgent need of blood transfusion, the date of the request, whether it is a reservation, the component type and the amount requested. The ID of the patient must be legible and complete, including the clinic and the bed, in addition to relevant clinical patient data. The diagnosis and the medications in use can help in reasoning for elucidation of possible positive reactions in imunohematológicas pré́-transfusion. The data for identification of the requesting physician also must be legible and contain the registration number in CRM.
- Note: you need to be careful, based on clinical and laboratory data, if necessary. The doctor should know the benefits and risks of each hemocomponente to be prompted by a haemoteraphists in the case of doubts.
- Routine after the start of transfusion: the patient must be monitored in 15 minutes. You may experience tremors, cyanosis, urticariformes reactions, pain in the path of the infusion, low back pain, hypotension, shock, dark urine, fever, tachycardia.
- Specific material: transfusion equipment with filter for retention of particles between 170 and mícras 22 or greater.
- Pré-transfusionais tests: tests are immuno-haematological studies made to select the hemocomponente compatible, in order to ensure a safe transfusion. They are: direct and reverse ABO typing and Rh (D) on the receiver; the presence of irregular antibodies in the recipient; and the proof of greater compatibility (GIRÃO et al., 2011). This process may take at least 40 minutes. With that, documents-if the urgency and the acceptance of a risk before the transfusion compatibility tests are finalized (MALIK et al., 2013).
Other precautions to be taken are: use the type O red blood cells when blood typing is unknown; When possible, Rh negative blood cells should be used in women of childbearing age to avoid the possibility of sensitivity when Antigen D (MALIK et al., 2013).
Made the initial considerations, and already described the processes of collection and separation of blood products, ressaltaremos in this part of the work the indications for therapeutic use.
12.1. Whole Blood (ST)
The ST has the capacity to carry oxygen, is plasma Expander and contains stable clotting factors (MALIK et al., 2013). The indication for treatment involving ST is for patients with active bleeding who have lost more than 25% of your total blood volume and can evolve to hemorrhagic shock (RAZOUK et al., 2004). In addition to this, the current indications are rare, is it brings advantages in the correction of anemia in relation to red blood cell concentrate, due to overload of the volume to be infused (GIRÃO et al., 2011). The development of blood products has restricted the use of ST. a few clinical conditions. The use of ST fresh is no longer considered accepted on hemotherapy and reveals only the lack of availability of appropriate products when used. Therefore, its use should only be raw material for the preparation of blood products (RAZOUK et al., 2004). For most cases of active bleeding in trauma and surgery, concentrated red blood cells and crystalloid solutions associated with Hemostatic elements, if necessary, are the treatment of choice (MALIK et al., 2013). A definitive contraindication to the use of the ST is the severe chronic anemia (RAZOUK et al., 2004).
In an adult, a unit of whole blood hemoglobin increases by approximately 1 g/dL or hematocrit in 3-4%. In children it is recommended if the transfusion of 8 mL/kg of ST. Must be administered through transfusion filter within 4 hours (HOFFBRAND et al., 2006).
When stored for more than 24 hours, contains few platelets, white blood cells and clotting factors viable, and rarely is available before the 24 hours, due to the time required for conducting serological tests and immuno-haematological studies. The validity is 35 days if using bags with preserver CPDA-1 anticoagulant solution. The broadcast time depends on the clinical condition of the patient, and may not exceed 4 hours (GIRÃO et al., 2011). When used, must always be identical ABO (MALIK et al., 2013).
12.2. Red cell concentrate (CH)
The transfusion of CH is intended to restore the ability of oxygen transport and the erythrocyte mass. So, your statement is related to the impairment of oxygen supply to the tissues, triggered by low levels of hemoglobin (Ministry of health, 2013).
In normovolêmicas anemia, especially in the slow installation, reduced oxygen transport capacity is compensated by:
- Increase in cardiac output (increased heart rate);
- Increase in the amount of 2.3-DPG (2.3-difosfoglicerato) red blood cells which leads to a deviation from the oxygen dissociation curve of hemoglobin. As a result of this increase, we observed a greater supply of oxygen at tissue level (Ministry of health, 2013)
Despite these compensatory changes, there are cases that they are insufficient. In these cases, indicated the erythrocyte mass replacement by CH transfusion (Ministry of health, 2013).
The anemias for acute bleeding, the replacement must be with crystalloid and/or synthetic plasma substitutes. The use of CH is reserved for situations in which the estimated blood loss was greater than 30% of blood volume (approximately 1,500 ml in adults). This assessment must be made by the medical team that is watching the patient (Ministry of health, 2013). A unit of red blood cells increases the adult hemoglobin 1 g/dL, hematocrit, and 3% (RAZOUK et al., 2004).
Some criteria for CH transfusion can be defined and used as a parameter for the indication of blood transfusion:
- HT 15% or Hb ≤ 5, 0 g/dl with chronic anemia and with no signs of tissue hypoxia (stable patients and may be subjected to situations that reduce oxygen consumption);
- HT ≤ 21% or Hb ≤ 7, 0 g/dl with acute anemia and with no signs of tissue hypoxia and without aggravating factors;
- HT ≤ 27% or Hb 9, 0 g/dl in patients with cardiovascular atherosclerosis without angina (often elderly or patients with chronic ischemic coronary disease) and clinically stable;
- HT ≤ 27% or Hb 9, 0 g/dl in patients with chronic lung disease or acute, with impairment of oxygenation (pO2 below 80mmHg);
- HT ≤ 27% or Hb 9, 0 g/dl in patients with acute tissue ischemia frames or increased consumption of oxygen by clinical condition (such as serious infections, post-operative of large surgical procedures etc.);
- HT ≤ 30% or Hb 10 g/dl in patients with ischemic cardiomyopathy in the immediate postoperative period of coronary artery bypass grafting;
- acute bleeding (blood loss exceeding 10 mL/kg in 1 hour) (Ministry of health, 2013).
12.3 platelet concentrate (PC)
Platelets have the function to control the bleeding by acting as a buffer in the hemostatic vascular endothelium. The use of platelets ABO compatible with the receiver it is recommended, but not compulsory, not requiring the achievement of compatibility tests pré-transfusionais (RAZOUK et al., 2004).
The clinical indications for transfusion of platelets are to prevent or control bleeding in patients with low platelet counts (thrombocytopenia), or, less often, in patients with platelet dysfunction (trombocitopatias) (RAZOUK et al., 2004). The indications for transfusion of CP are associated with bone marrow failure triggered plaquetopenias. Rarely, we indicate the replacement in plaquetopenias by peripheral destruction or congenital or acquired changes of platelet function (Ministry of health, 2013). The indication of platelet transfusion should be based on the cause of the bleeding, the patient's medical conditions, in addition to the number and function of circulating platelets. The indications may be therapeutic and/or prophylactic according to the following list:
- Prophylactic
- Patients trombogênicos (count below 50000/mm ³) which will be submitted to surgery or invasive procedures, such as for example: epidural anesthesia, transbronchial biopsy, liver biopsy, laparotomy, deep veins puncture, paracentesis and thoracentesis, tooth extraction, gastric biopsy.
- Neurological and ophthalmic surgeries: it is recommended that the platelet count is around 100000/mm ³
- Cardiac surgeries with cardiopulmonary bypass: there is no consensus in the literature about the minimum count of 50000 or 100000/mm ³.
- Bone marrow biopsy, spinal tap and Bronchoscopy (without biopsy): it is recommended that the platelet count is above 20000/mm ³.
- Bone marrow Aplasia post-chemotherapy and/or radiotherapy: displayed when the platelet count is less than 10000/mm ³ than 50000/mm ³ before invasive procedures. In patients who have risk factors for bleeding, as major esplenomegalias, fever, antibiotics or antifungals, this trigger can be higher (15 or even 20000 platelets/mm ³).
- Aplásica anemia and myelodysplasia: there is no consensus that the prophylactic platelet transfusion in patients with stable chronic thrombocytopenia due to production, reduce the occurrence of severe bleedings.
- Immune Thrombocytopenic Purpura (ITP): no indication of prophylactic platelet transfusion. In preparation for Splenectomy, transfuse if there is significant bleeding.
- Trombocitopenias secondary to hiperplenismo: it is recommended prior to invasive procedures.
- Trombocitopatias: it is recommended prior to invasive procedures (MALIK et al., 2013).
- Therapy
Is indicated for patients who are platelet dysfunction and hemorrhage with risk of death, independent of platelet count, and patients who have ongoing bleeding and platelet count less than 50000/mm ³ (MALIK et al., 2013).
- Special situations:
- In thrombocytopenia (immune Thrombocytopenic Purpura-PTI, dengue hemorrhagic fever and hypersplenism) and trombocitopatias: the transfusion is indicated in the presence of active hemorrhage with risk of death and suspected intracranial hemorrhage.
- Cardiac surgery: the surgery with cardiopulmonary bypass the transfusion is indicated in patients who present diffuse bleeding, independent of the platelet count. In the immediate postoperative period, the transfusion is indicated when there is bleeding and the platelet count is less than 50000/mm ³ or diffuse bleeding regardless of platelet count.
- Disseminated intravascular coagulation (DIC): the transfusion is indicates only the presence of active bleeding (MALIK et al., 2013).
The contraindications to Platelet transfusion are:
- Hemolytic uremic syndrome.
- HELPP Syndrome.
- Purple pós-transfusional.
- Stable immune Thrombocytopenic Purpura.
- Thrombotic thrombocytopenic purpura (TTP).
- Heparin-induced thrombocytopenia (MALIK et al., 2013).
13. Ffp (PFC)
In case of deficiency of a single coagulation factor, só́ should be used if you don't have available purified product (hemoderivado), because it has greater security. These situations are very infrequent and the spare indicator should always be associated with the presence of bleeding or risk of this in during any invasive procedure. There is also indication of use of PFCS in thrombotic risk situations as in the case of deficiency of Factor XI (FXI) (Ministry of health, 2013).
In the case of multiple deficiency of coagulation factors, should always be associated with the presence of bleeding or increased risk for this. Examples include: severe hepatocítica deficiency, disseminated intravascular coagulation, massive transfusion, etc. (Ministry of health, 2013).
The impairment of hemostasis happens when the deficiency of coagulation factors or factor is severe, resulting in a less than 30% – 40% (Ministry of health, 2013).
Most common situations:
- Disseminated intravascular coagulopathy (DIC) with bleeding;
- Immediate reversal of the effects of dicumarínicos (oral anticoagulation by antagonize vitamin K and decrease the synthesis of factors II, VII, IX and X), by bleeding or need for surgical procedure;
- Thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) as a replacement product in the therapeutic plasmaféreses;
- Severe liver disease with severe deficiency of coagulation factors in the presence of bleeding or need for surgical procedure;
- Replacement of clotting factors in situations of massive transfusion;
- Deficiency of Antithrombin-III (AT-III) (Ministry of health, 2013).
PFC should never be used only for volume expansion, or as nutritional support (protein replacement) in patients hipoalbuminêmicos (Ministry of health, 2013). This orientation because safer products, which offer no risk of disease transmission or allergic reactions to receivers, are available for this purpose, such as serum albumin, synthetic Colloids and balanced solutions solutions of saline (RAZOUK et al., 2004).
The doses often indicated to reverse the deficit of coagulation factor (s) is from 10 ml to 20 mL/kg of weight, rapid infusion. Smaller doses appear to be ineffective and should not be used (HOFFBRAND et al., 2006). Administration of doses at regular intervals of PFC as maintenance has been used, but without relevant evidence in the literature. Só́ should be considered in situations where there is the perpetuation of the causes (for example, disseminated intravascular coagulopathy) or bleeding, after use of the initial dose (Ministry of health, 2013).
It should be remembered that the significant impairment of hemostasis, with increased risk of bleeding in surgical procedures and/or invasive só́ occurs if there is a change of routine coagulation tests (values greater than 1.5 time the normal/default value). The use of PFC is not required, the independent laboratory evaluation, if the patient is not showing hemorrhagic manifestation or there is the need of surgical procedure and/or invasive (Ministry of health, 2013).
The ABO compatibility should be respected in the administration of fresh frozen plasma, with the aim of avoiding hemolysis in the receiver, although you are not required compatibility tests (RAZOUK et al., 2004).
14. Crioceptado (CREATE)
The main indications of the Cryo transfusion are in haemophilia treatment of von Willebrand's disease, congenital or acquired Fibrinogen deficiency, deficiency of factor XIII and obstetric complications or other situations associated with the consumption of Fibrinogen, as DLC (HOFFBRAND et al., 2006). Its use is also beneficial in the treatment of hemorrhagic tendency associated with uremia. In the case of hemophilia A, in Brazil there is a standardization of the Ministry of health, where the hemophiliacs should be treated only with factor VIII industrialized. Small amounts of CREATE are used in the preparation of "fibrin glue" for the aid of surgical hemostasis or for removal of kidney stones (RAZOUK et al., 2004).
In heart surgeries, patients with platelet dysfunction due to kidney failure may benefit from the pre-surgical transfusion of CREATE, due to increased von Willebrand factor. Postoperatively, the infusion of CREATE is indicated in the bleeding due to hipofibrinogenemia. Should be administered at a dose of a purse/10 kg of patient weight (RAZOUK et al., 2004).
The CREATE should not be used in the treatment of patients with deficiencies of other factors other than those already mentioned. The risk of transmission of infectious diseases, for each unit to CREATE, is the same of the PFC. When high amounts of CREATE are transfused, the Fibrinogen level of the individual must be monitored because it can reach very high levels (hiperfibrinogenemia), leading to an increased risk of thromboembolism (RAZOUK et al., 2004).
If the title of isoagglutinins exceeds 1:32, one must respect the ABO compatibility, especially in children, because one can observe a positive direct Coombs test in the receiver which receives ABO incompatible units (HOFFBRAND et al., 2006).
15. Transfusion reactions
According to the art. 6, Ordinance No. 2,712, of 12 November 2013 the Ministry of health "the blood transfusion and its components must be used judiciously in medicine, since all transfusion brings in itself a risk to the recipient, whether immediate or delayed, should be given judiciously." That's because, even under the best conditions the blood transfusion is associated with any complications. The frequency and severity of many types of reactions are difficult to determine with accuracy and probably vary from one institution to another. (SMELTZER et al., 2005)
It is known that transfusions of blood products were never as safe as now, however, as with any medical procedure, in some situations you may experience adverse transfusion reactions, whose recognition is important for therapeutic measures are established, as well as prevention strategies for future transfusions. (HAMERCHLAK et al., 2010)
Is called transfusion reaction to any sign or symptom that occurs at the beginning, during or after the transfusion of blood products. In Brazil, it is set to immediately when less than 24 hours after the transfusion, and late when it occurs after this period and may be diagnosed several years after the transfusion (Forbes et al., 2011); (HAMERCHLAK et al., 2010)
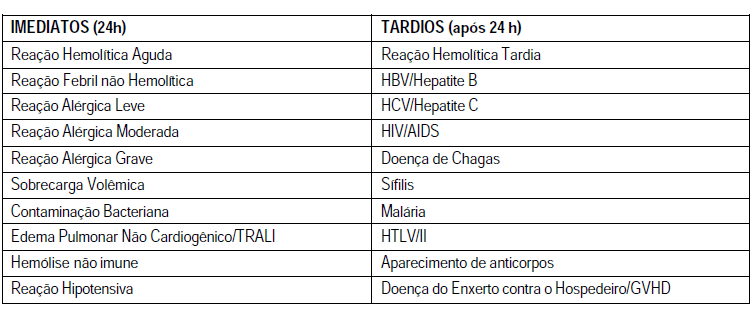
15.1. Main immediate transfusion reactions
The major signs and symptoms associated with that lead to suspicion of a possible acute transfusion reaction are: fever with or without shaking, defined as the increase of 1° C body temperature; shakes and chills, with or without fever; infusion site pain, chest, abdomen and flanks; change in blood pressure: hypertension or hypotension, flushing, Erythema, urticaria or edema or located, nausea with or without vomiting, and change in color of urine being that this may be the earliest sign of an acute hemolytic reaction in patients anesthetized (BARRETO et al., 2011).
Hemolytic transfusion reactions occur when transfused red blood cells are destroyed, and can be immediate or late. Immediate reactions, with risk of death associated with intravascular hemolysis, resulting from the action of activators of complement antibodies IgM and IgG, usually with specificity ABO. (HOFFBRAND et al., 2013) According to its nature, can be divided into two groups: intravascular hemolytic reaction whose main cause is the ABO incompatibility, which almost always results from human errors, such as pre transfusion samples barely identified, misidentification of blood after the cross or Exchange at the time of installation, in which the receiver usually features lumbar pain intense in the first few minutes after the blood transfusion and may present also fever (with or without chills), hypotension, nausea, dispinéia and feeling of imminent death; And extravascular hemolytic reaction manifested by fever and abdominal or back pain of mild to moderate intensity, which arise usually from 30 to 120 minutes after the transfusion. (HEMORIO, 2012). In the case of suspected hemolytic reaction, new blood samples will be collected from the receiver that will be labeled appropriately and, along with the bag of blood component in question, even empty, will be immediately forwarded to the Hematology service. Pré-transfusionais tests shall be repeated with the samples before and after the tranfusional reaction (Ordinance No. 2,712, page 49).
Febrile non-hemolytic reaction (RFNH), is defined as the temperature increase of 1° C associated with the transfusion without any other explanation. The definition of 1 C is arbitrary, since the same events can cause larger temperature increments. This increase can be immediate or delayed (starting after several hours of the completion of the transfusion). Situations that may lead to aloimunização, especially pregnancy and multiple blood transfusions, increase the frequency of RFNH. (BARRETO et al., 2011) The specific procedures for fever; above 37° C in patients previously afebris, or elevation greater than 1 C in a patient previously with fever; or chills are: suspend the transfusion and request the tests for the investigation of transfusion reaction, prescribe antipyretic parenteral, intravenous, ask the nurses to collect the bag of blood, being careful to isolate the end of equipo (lid, clamp or node) that was connected to the patient's vein. This procedure aims at realization of microbiological culture on hemocomponente, and request the sample collection (s) of patient's blood to perform blood culture, where necessary. (HEMORIO, 2012)
Allergic reactions are usually caused by hypersensitivity to plasma proteins of the donor or by exposure to substances present in the plasma of donors, for which the receiver was already aware. (HOFFBRAND et al., 2013). Can be divided into three stages according to the severity of the clinical manifestations: mild, moderate and acute. A mild allergic reaction is characterized by itching, rash and erythematous plaques; and occurs in a the 3% of blood transfusions. If occurs only hives, the transfusion can be stopped temporarily, while the antihistamine is administered orally or parenterally. Mild symptoms are quickly reverted and can be prevented by the administration of antihistamines before transfusions. (BARRETO et al., 2011). Already the moderate allergic reaction is characterized by the occurrence of edema of glottis oedema Quincke and bronchospasm; While the is characterized by acute anaphylactic shock. (HEMORIO, 2012). Anaphylaxis usually occurs at the beginning of the transfusion, with systemic symptoms that often are severe as loss of consciousness, shock and, in rare cases, death. Symptoms may involve one or several systems, notably the respiratory tract (cough, bronco spasm, Dyspnea), gastrointestinal tract (nausea, vomiting, and diarrhoea), circulatory system (arrhythmias, hypotension, syncope) and skin (rash generalizes the, urticaria). These manifestations seem widespread reflex activity of IgE antibodies, although it may not be demonstrated in the serum of many patients. Receivers that feature with frequency urticariformes associated with transfusion reactions, can respond well to the administration of antihistamines before transfusions. If the reactions are applicants, especially severe, should be transfused blood products. (BARRETO et al., 2011). In cases of moderate and severe reactions should suspend the transfusion and prescribe intravenous steroids. If there is bronchospasm nebulizer should be prescribing with bronquiodilatadores, and if the reaction gets worse or do not improve with treatment, prescribed adrenaline. In severe reactions, with anaphylactic shock, it must suspend the transfusion immediately and adopt as a therapeutic application of adrenaline in five-minute intervals until satisfactory answer. (HEMORIO, 2012)
The transfusion-related acute lung injury or acute non-cardiogenic pulmonary edema (TRALI), is as the name says, a transfusion-related acute lung injury. Can be of moderate to severe and usually grows from 2 to 6:00 after the transfusion due to transfusion of anti-HLA class I and II antibodies present in the donor's plasma and/or granulocíticos specific antigens. These antibodies bind to the antigens of receptor, triggering immune events that increase the permeability of the pulmonary microcirculation and allow the passage of fluid into the alveoli. (HEMORIO) Specific antibodies can be absent and some cases of TRALI apparently result from other mechanisms. The activation of complement C3a and C5a can generate anafilotoxinas, leading to aggregation of granulocytes, which lodge in the pulmonary microvasculature. Recently, reactive from lipid products of cell membranes in the donor blood products have been linked to the etiology of TRALI. The treatment is based on reversal of hypoxemia with oxygen and ventilatory assistance if necessary (BARRETO et al., 2011). If the transfusion has not finished it should be suspended. Mortality is around 6 to 14%. However, with intensive care, most patients recover lung function of 72 to 96 h after the beginning of treatment (HEMORIO, 2012).
Volume overload or hypervolemia must be considered when the patient: dyspnea, cyanosis, severe headache, hypertension, or congestive heart failure, during or soon after the transfusion, due to overload of the circulatory volume. Children and the elderly are considered to be the population most at risk. The rapid increase in volume is little tolerated by patients with commitment of cardiac and/or pulmonary functions and/or with chronic anemia. The symptoms improve when the transfusion is suspended and the patient is placed in a sitting position. Diuretics and oxygen are often applied. If your symptoms do not subside, Phlebotomy can be indicated. Patients with risk of volume overload should receive red blood cells concentrate pós-transfusional slowly at a dose of 1 ml/Kg/hour. If the desired amount exceed the maximum period of 4 hours, it is prudent to transfuse in tax rates according to the needs of the patient. The administration of diuretics before and during the transfusion can be useful. (BARRETO et al., 2011)
The bacterial contamination are the leading cause of morbidity and mortality related to transfusion, especially when you consider transfusions of platelet concentrates (Apheresis or random). Bacteria contaminants, mostly originate from the donor, is the site of venipuncture or as a result of bacteremia. Bacterial multiplication is sharper in components stored at room temperature (platelets), and Gram-positive bacteria isolated in these components; the gram-negative bacteria are isolated in components stored under refrigeration (units of blood). (BARRETO et al., 2011)
Non-immune hemolysis can occur when red blood cells are frozen or overheated; When there is concomitant administration of medications and/or hydration (dextrose), when the blood is administered under pressure (cardiopulmonary bypass) or when there is violent handling of blood (HEMORIO, 2012).
Bacterial reactions are rare, but have a high rate of mortality, characterized by fever, chills, breathlessness, hypotension and disseminated intravascular coagulation. (HAMERCHLAK et al., 2010) Bacterial multiplication in the bag leads to oxygen uptake, resulting in hemoglobin desaturation and erythrocyte Lysis, resulting in change of colouring of the purse, formation of clots or hemolysis and suggesting how the contamination (BARRETO et al., 2011). The main contaminants are Staphylococcus spp and Serratia spp, exogenous bacteria of species; Enterobacteriasceae, family of bacteria found in the human gut, in soil and water; Treponema pallidum cause syphilis and Borrelia burdorferi, the cause of Lyme disease (HOFFBRAND et al., 2013).
The pipeline consists of contamination to interrupt immediately the transfusion, collect the material bag for blood culture, if possible, perform Gram stain of the material of the bag, collect material from the receiver the transfusion, for blood culture, administer the symptomatic receiver and antibiotics as medical assessment. To prevent such a reaction the important first step is a careful selection of blood donors, held through the interview, proper selection and meticulous cleaning of the puncture site donor-preparing the skin of this does not eliminate the risk of contamination, but reduces important-dispose of first rate of blood collection, using this material to achieve the complementary exams of screening care related to the handling of blood products transfusion agencies and realization of microbiological quality control of blood products in routine (BARRETO et al., 2011).
15.2. Main transfusional reactions late:
Late hemolytic reactions can occur when the concentrated red blood cells transfusion induces an immune response against erythrocyte days or weeks after the transfusion. Studying with indolent clinical picture, often malaise, weakness, anemia and jaundice, but does not cause risk to the patient. Typically, acute therapy is not required, but care in the selection of packed red blood cells compatible for future transfusions are essential to prevent new reactions. Are usually involved other antibodies blood systems than the ABO, Rh and Kell Systems (HAMERCHLAK et al., 2010).
Despite the technological advances in testing for infectious agents, the risk of transmission of viruses, bacteria or protozoa still persists. These agents are characteristic: persistence in the bloodstream, generating diseases with latency stage; population likely well known; ability to cause asymptomatic infections; stability in stored blood and, in some cases, during the plasma fractionation. The collected blood should be tested for pathogens prevalent in the population that may cause serious illnesses. For both tests are used for mass screening, characterized by its high sensitivity.
However, even using high-sensitivity tests, not all agents are detected, given the technical limitations of the tests, the existence of the immunological window and mutant forms of pathogens. The decrease of the risk passes, in addition to the residual transfusion screening serological, also by a rigorous selection of donors through the screening clinic. (BARRETO et al., 2011) In Brazil, the gatehouse 1376/93, reinforced by resolution nº 343 MS/2001, determines the serological screening tests in the Hematology services for syphilis, Chagas disease, hepatitis B and C, HTLV and HIV/Aids, malaria. As for recipients of blood, this Ordinance determines the immuno-haematological tests, namely, ABO/Rh, irregular antibodies (FATHER) and compatibility tests (Cross).
When the serological screening tests become positive in a donor with prior donation and serology, reagent or not in a patient who received a blood transfusion, is what we call seroconversion (donor and receiver, respectively). In these cases, should be started the retro surveillance process, characterized by the identification of all blood products, donors and recipients involved in the case, using tracking tools and retestagem to conclude about the association between blood transfusion and further (BARRETO et al., 2011).
Some viral infections such as hepatitis (B, C, D), human immunodeficiency virus (HIV), Cytomegalovirus (CMV), Herpes Virus 6 and 8, and Parvovirus B19, can be transmitted by transfusion of blood products. (HAMERCHLAK et al., 2010)
Donors with a history of hepatitis are rejected for 12 months. If there is a history of jaundice can be accepted if the markers for hepatitis B and hepatitis C are negative. Have HIV can be transmitted by plasma cells. Gay men, bisexuals, illicit intravenous drug users and prostitutes are excluded, as well as their sexual partners and partners of hemophiliacs; as a form of prevention. A rare event that transmission occurs when the donor is already infected, the incubation, and is still not positive for the HIV antibody screening test used in triage: donation made on immunological window (HOFFBRAND et al., 2013).
The Cytomegalovirus infection (CMV) is often subclinical, but can present itself as a syndrome of Mono. In immunosuppressed individuals, the infection can cause potentially fatal Pneumonitis. Under this risk are premature babies, containers of stem cells and transplantation of other organs and pregnant women (risk to the fetus). On the other hand, syphilis is transmitted more easily by platelet concentrate than blood (HOFFBRAND et al., 2013).
There are also reports of protozoa transmitted by transfusion. They are: Plasmodium spp. (malaria), Trypanosoma cruzi (Chagas disease), Toxoplasma gondii (toxoplasmosis) and Leishmania spp (leishmaniasis) (HOFFBRAND et al., 2013). According to the art. 210, Ordinance No. 2,712, of 12 November 2013 the Ministry of health all cases where there is suspicion of infection transmission by transfusion will be evaluated from a new study of donors of blood components suspected, including the convening and the repetition of tests for communicable infections of all donors involved. After you identify the donor, it is forwarded to specialized treatment and deleted from the donors of the Hematology service.
Human leukemia viruses in T cells can be detected during screening. The virus type I (HTLV-i) is associated with adult T-cell leukemia or paraparesia tropical espática. The virus type II (HTLV-II) no known association with any clinical condition (HOFFBRAND et al., 2013).
The graft-versus-host disease associated with blood transfusion is a complication associated with transfusion fatal immune clonal expansion of lymphocytes from the donor in a susceptible host. The transfused lymphocytes multiply in a receiver likely tissue, leading to pancytopenia refractory, with bleeding and infectious complications, which are responsible primarily for 90%-100% of the mortality rate of patients affected. So far, there is no effective treatment for the disease; so, emphasizes the necessity of prevention which consists of gamma irradiation of cellular components. The minimum dose standard is 2500cGy. This dose makes lymphocytes capable of replication, without affecting substantially other cellular components. (BARRETO et al., 2011)
16. The problem of underreporting
Control of the hemotherapy in Brazil faces a big problem due to low notification of cases of transfusion reactions. Some States like Mato Grosso until a few years ago had not notified if you want a case according to figures released by the national agency of sanitary surveillance (ANVISA, 2014, p 10), but the problem persists, because even in the year in which the largest number of notified cases that not even the 20% of the actual amount of estimated reactions by ANVISA (2014 , p. 18).
17. State of Mato Grosso
The Mato Grosso has 17 units of collection and transfusion (Prod), 30 Transfusional Agencies (AT) and only one blood donation Coordinator who is located in the capital. These units meet the 13 micro-regions, each unit fulfilling a specific function, the ATs only blood transfundem, while the transfundirem Prod as well are empowered to receive also the hemocentro blood donation Coordinator, in turn, is responsible for all the analyses of qualification of the blood tissue (BLOOD, 20110, p. 1-2). This centralization ends up generating multiple disorders as the blood is not able to perform with high speed tests for determination of the conditions of the material collected resulting in situations like that of Sinop where units need to be discarded for salary according to the FOLHAMAX newspaper. Considering the number of donations in the State that, in 2012, were of 77,802. (Ministry of health, 2012, p. 26)
In relation to consumption percentages, the majority goes to the medical clinic (more than 45%) followed by surgical clinic, Pediatrics, obstetrics, long-term care that corresponds to less than 5% and, finally, the tisiologia. As for the blood products red blood cells concentrate leads the ranking of blood transfusions which corresponds to approximately 50% of transfused platelet concentrate followed (23.01%) and fresh plasma with 18.86%, and platelets by Apheresis those of less use (Ministry of health, 2012, p. 58).
Conclusion
The blood transfusion is a complex process since to ensure safety not just remove from the donor and transfuse on the receiver. The security measures begin in the collection through a donor screening, undergoing laboratory tests of donated tissue, separation of blood products, selection of recipients and the specific material being transfused until, finally, the process. Nevertheless many reactions can still be observed not doing, however, this Act an amazing display of charity and a procedure that saves milhões people every year.
Bibliographical References
- ANVISA JOINT TECHNICAL NOTE n° 001/GGPBS/GGMON/CGSH 2015
- HEMOVIGILÂNICA BULLETIN No. 06/2014
- BATISSOCO, Ana Carla And Marcia Cristina NOVARETTI Zago. Molecular aspects of ABO Blood System. Rev. Bras. Hematol. Hemoter. 2003, vol. 25, no. 1, pp. 47-58.
- Ministry of health. The national health surveillance agency. Technical manual for investigation of the transmission of diseases by blood. Brasília-DF, 2004;
- Ministry of health. Secretariat for health care. Department of Specialized Attention. Guide to the use of blood products. Brasília-DF, 2008.
- Borges, D.R. therapeutic Update of Prado, Ramos and Valle: diagnosis and treatment-2014/15. 25 ed revised and current-São Paulo: medical arts, 2014.
- CARRAZONE, Cristina. GARCIA, Yara m. importance of evaluation sorofisiológica pre-transfusion levels in blood recipients. Rev. bras. Hematology and hemotherapy. paragraph 26, PG. 93-98, 2004.
- COLSAN, blood collection Benevolent Association-Hematoterapia guide 2011
- Manual of Haemotherapy, 7ed., 2011.
- Technical criteria for health risk management in the use of blood products transfusion procedures vis-à-vis the situation
of Public Health Emergencies of national importance for cases of Zika Virus infection in Brazil. - State is sentenced to perform accreditation of blood bank I Sinop. FolhaMax, Cuiaba, 21 Sept. 2015. Available at: <http: www.folhamax.com.br/cidades/estado-e-condenado-a-realizar-credenciamento-de-banco-de-sangue-em-sinop/60191="">accessed: 24 February 2016.</http:>
- STRONG, Hildenete. Transfusion request: routine and emergency. Cuiabá: University Hospital Julius Muller. Page 1-4, 2003.
- FIGUEIREDO, M.S.; KERBAUY, J.; LAWRENCE, D.M. Guide to hematology. Number outpatient and hospital medicine guides of EPM-UNIFESP. Barueri, SP: Manole, 2011.
- BOTTLE, Volnei. AZAMBUJA, Leticia Erig Osório. Jehovah's witnesses before the use of blood products, and blood products. Medical Association Magazine. n° 56. page. 705-709, 2010.
- GINGERICH, D.A. Fluid, shock and blood therapy. In: HOWARD, J.L. Current veterinary therapy. Food animal practice 2. Philadelphia, W.B. Saunders Company, 1986. p. 1-8.
- S. Malik, m. c. t. Daly, s. b. Awada: Er: emergency medicine. revised and expanded 3Ed, Manole, 2013.
- HAMERSCHLAK, n. Hematology Manual: integrated programme of Hematology and bone marrow transplantation. Barueri, SP: Manole, 2010.
- rj.gov.br
- Hoc, Hospital Oswaldo Cruz Institute. Step-by-step blood donation. Paradise. 2015.
- HOFFBRAND, A. V.; MOSS, p. a. h. Fundamentals in hematology. 6. Ed. Porto Alegre: New Haven, 2012. 464p.
- HOFFBRAND, A.V.; MOSS, P.A.H. Fundamentals in hematology. 6 eds. Porto Alegre: New Haven, 2013.
- HOSGOOD, g. Blood transfusion: A historical review. J. Am. Vet. Med. Assoc., v. 197, no. 8, pp. 998-1000, 1990.
- JUNQUEIRA, L.C.U. & CARNEIRO, J. Basic Histology. 11th Ed. Rio de Janeiro: Guanabara Koogan, 2008.
- Junqueira, p. c. history of hemotherapy in Brazil. Rev. Bras. Hematol. Hemoter. Vol. 27. São José do Rio Preto. Sept. 2005.
- Ludwig, s. t. blood donation: a marketing vision. CAD. Saúde Pública, Rio de Janeiro, may-jun, 2005.
- MANUAL OF HOSPITAL TRANSFUSION AND TRANSFUSION COMPLICATION. Curitiba: Hematology and hemotherapy Center of Paraná-Hemepar. v. 1. Page 9, 2013.
- MANUAL FOR RATIONAL USE OF BLOOD. Florianópolis: Federal University of Santa Catarina. HU/UFSC. Page. 21, 2011.
- MINISTRY OF HEALTH. Product information: blood and blood products. 2012, p 10 and 18.
- THE GLOBE. Jaqueline Hawk. Available in <oglobo.globo.com ociedade/saude/paciente-foi-infectado-com-virus-zika-por-transfusao-de-sangue-18315625="">.</oglobo.globo.com> Access in: 2/9/2016
- THE GLOBE. The globe with international sites. Available in <oglobo.globo.com ociedade/saude/sangue-sintetico-podera-ser-testado-em-2017-diz-servico-de-saude-britanico-16550752="">.</oglobo.globo.com> Access in 2/23/2016.
- Clinical use of Blood. Geneva. p. 1-162. 2013.
- JEHOVAH'S WITNESS ORGANIZATION. Don A. Adams. Available in <www.jw.org t/testemunhas-de-jeova/perguntas-frequentes/por-que-testemunhas-jeova-nao-transfusao-sangue/="">.</www.jw.org> Access in: 2/9/2016.
- MINISTRY OF HEALTH ORDINANCE n° 1,353, give 1 13.06.2011 of 14.06.2011. Approving the technical regulation of Hemoterápicos Procedures
- MINISTERIAL ORDER n° 2,712, of 12 November 2013. Official Gazette n° 221, of 12 November 2013, Section 1, PG. 106.
- Ordinance No. 2,712, of 12 NOVEMBER 2013
- PRO BLOOD. Pr. Dr. Vicente Odone Son. Available in <www.prosangue.sp.gov.br ome/default.aspx="">.</www.prosangue.sp.gov.br> Access in: 2/9/2016
- Proceedings from the National Summit on Overuse, 2013. The Joint Commission. Available at: http://jointcomission.org/overuse_summit/.Accessed May 31, 2014.
- Published in Official Gazette No. 221, of 13 November 2013, Section 1, page 106.
- Frostbite: diagnosis and initial treatment/Brazilian Society of plastic surgery-Brasilia: Brazilian Medical Association and Federal Council of Medicine, 2008. 14 p.
- RAMOS E SILVA, M.; CASTRO, M.C.R. Dermatology fundamentals. Revised and updated edition. Rio de Janeiro: Atheneu, 2010. Vol 1.
- RAZOUK, f., et al. Characterization, production and clinical indication of the main blood products. Rev Brazilian of Hematology and hemotherapy. 2004; 26 (2): 126-134.
- ROSENFELD, r. foundations of CBC: from the lab to the clinic. 1 ed. Rio de Janeiro: Guanabara Koogan, 2007.
- SAKABE, d., et al. Volume replacement in patients patients. Rev of the Faculdade de Ciências Médicas de Sorocaba, v. 6, n. 1, p. 21-28, 2004.
- SCHMOTZER, W.B. et al. Time-saving techniques for collection, storage and administration of equine blood and plasma. Vet. Med., v. 80, n. 2, pp. 89-94, 1985.
- Section XIII-Transfusion Reactions
- SMELTZER, S. C.; BARE, B.G. Brunner and Suddarth: Medical-Surgical Nursing Treaty. 2, 10° Edition, Rio de Janeiro: Guanabara Koogan. 2005.
- Hematology technician: textbook/Ministry of health, Secretariat of management of Work and education in health, Department of Health Education Management – Brasília: Ministry of health, 2013. 292 p.
- Sybille de La Hamaide and Myriam Rive. Available in noticias.uol.com.br/ultimas-news/reuters </2016/02/07/France-restricted
doacao-de-sangue-por-causa-do-zika-virus.htm >. Access in: 2/9/2016 - HOFFBRAND, P. A. H. MOSS, J. E. PETTIT. Fundamentals in hematology. 5th ed. Rio de Janeiro, New Haven, 2006.
- VERRASTRO, THEREZINHA. Hematology and hemotherapy: Fundamentals of Morphology, Physiology, pathology and clinic. 1st ed. São Paulo: Atheneu, 2006.
- Marcelo Vieira da Silva. Genetic approach and imunofisiológica of the ABO and RH systems for better understanding and teaching of Erythroblastosis fetalis. Belo Horizonte, 2013.
[1] Master's degree in Pharmacology from the Faculty of Medicine of Ribeirão Preto (FMRP) da Universidade de São Paulo, USP (1993); Doctor of health and environment, the area of concentration of Natural Products in the Pharmacology Institute of collective health (ISC) of the UFMT (2001). Bachelor's degree in law from the UFMT (2001). Lawyer. Has experience in the area of health law, Biolaw, doctor and hospital Law, primary health care, Public Health Policy, epidemiology, Pharmacology (with emphasis on Pharmacology of the inflammatory process and smooth muscle Pharmacology), Pharmacoeconomics, Pharmacoepidemiology. Contact: [email protected]















