ORIGINAL ARTICLE
CASALOTI, Laís Guadalupe [1], SANTOS, Jorge Alexandre Nogueira [2]
CASALOTI, Laís Guadalupe. SANTOS, Jorge Alexandre Nogueira. Inhibition of elastase by aqueous and ethanolic foliar extract of Sedum Dendroideum: An in vitro study. Revista Científica Multidisciplinar Núcleo do Conhecimento. 04 year, Ed. 07, Vol. 04, pp. 140-147. July 2019. ISSN: 2448-0959
SUMMARY
The unregulated activity of proteases in humans is related to a number of diseases. Neutrophil Elastase is a protease controlled by the α1-antitrypsin inhibitor (AAT) that is naturally produced by the liver. Mutations in the SERPINA 1 gene cause deficiency of this inhibitor and the consequence of this is the emergence of several pathologies in humans. Given that plant species are promising sources for the discovery of new drugs, the present study aimed to evaluate the inhibitory effect of the extracts on elastase, where IC50 values of 8.1 ± 1.4 µg/ml (aqueous extract) and 3.3 ± 0.6 µg/ml (ethanolic extract). These results suggest that the extracts of Sedum dendroideum may be a potential source of bioactive compounds for the discovery of new inhibitors for elastase.
Keywords: Balm, IC50 inhibitors.
INTRODUCTION
Proteases are essential enzymes for a large number of physiological processes such as protein digestion, reproduction, blood coagulation, Kallikrein-Kinin system and Fibrinolysis (Otín & Bonde, 2008). In humans, the unregulated activity of proteases is related to various pathological conditions such as cancer, inflammatory processes, thrombosis, arthritis, skin diseases and pulmonary emphysema. For this reason the proteases activity needs to be controlled and regulated (Rawlings et al., 2004). Recent research has revealed that proteases have become important targets for drug development. Elastase is a protease produced mainly by the pancreas and neutrophils, and it has the property of hydrolysing components of the extracellular matrix, such as collagen, elastin, Laminin and Proteoglylians (Thomson & Kapadia, 1979). The proteolytic activity of neutrophilic elastase is strictly regulated by the endogenous protein inhibitor called α-1-antitrypsin (AAT). Mutations in the SERPINA 1 gene, Locus Pi, located on chromosome 14 (14q31-32) cause AAT deficiency (Siedle, et al., 2003). Without the presence of its natural inhibitor, the elastase in abundance generates tissue lesions, since this enzyme is the main protease released by neutrophils in inflammatory processes.
The action of Elastase is related to a series of diseases such as liver failure, rheumatoid arthritis, psoriasis, skin cancer, arteriosclerosis and several pulmonary pathologies (Johansson, 2002). In a normal lung, the alveoli are exposed to low levels of neutrophil elastase. This protease can destroy the elastin in the alveolar wall if it is not contained by the action of the α-1-antitrypsin inhibitor. After injury, the lung tissue cannot regenerate and a process of emphysema, asthma, acute lung injury or cystic fibrosis can be triggered (Abboud & Vimalanathan, 2008).
The prospecting of bioactive compounds from natural products finds in the plant species the main and most promising source of new compounds that can act as protease inhibitors. To protect against herbivorous animals and insects, plants usually produce secondary metabolites that include terpenes, polyphenols, tannins, peptides and proteins (Ibanez et al., 2012). Many of these metabolites are inhibitors of proteases and cause a decrease in the absorption process of essential amino acids for the development of insects (Cuccilioni et al., 2009).
The species Sedum Dendroideum, popularly known as Balsam is a plant belonging to the order Saxifrales, Moc. Et Sessé ex DC, family Crasulaceae is a perennial, succulent, sublenhosa and xerophyte species, originating in South Africa, from the dry tropical climate (Epagri,1998). In Brazil, it is widely adapted and is popularly called Balsam (Milaneze & Gonçalves, 2001). The juice of its leaves has been used for the treatment of cutaneous inflamation and bruising, and internally for gastric disturbances, due to emollient and healing activities (Epagri, 1998; Milaneze & Gonçalvez, 2001).
Studies indicate that the genus Sedum presents compounds of different chemical groups such as polysaccharides (Sendl et al., 1993), tannins (Stevens et al., 1995), Triterpenoids with hepatoprotective activity (Aimin, et al. ,1998), piperidinic alkaloids (Halin et al., 1985) and Pyrrolidyic (Harth et al., 1996). And through a phytochemical analysis with the species Sedum dendroideum it was possible to identify the presence of Kaempferol, an important molecule with pharmacological properties (Coutinho, Muzitano & Costa, 2009). Taking into account the existence of secondary metabolites found in the species, and in view of its potential use as a medicinal plant, the present study aimed to verify whether the aqueous and ethanolic extracts of the plant have potential for Inhibition on the proteolytic activity of the elastase enzyme.
MATERIALS AND METHODS
The extracts were prepared from the leaves of Sedum Dendroideum following the methodology of Heidari-Sureshjani (2015), in which fresh and healthy leaves of the vegetable were collected in the neighborhood Furnas (latitude 22° 26′ 27″ and longitude 46° 21′ 03″), located in Municipality of Bueno Brandão.
About 10 g of the leaves of the plant were dried for 72 hours in a greenhouse at 40 ° C and subsequently grinded until obtaining a uniform-looking powder. Then, the material obtained was transferred to a glass vial and mixed with 50 ml of distilled water, remaining at rest for 12 hours. After this procedure, the mixture was filtered on the Whatman paper No. 1 and a clear, particle-free liquid was obtained. The same procedure was used for the preparation of Ethanolic extract, however in the place of distilled water was used ethanol 95%.
For the inhibition assays, the enzyme elastase of the pig pancreas (E. C 3.4.21.36, ≥ 4 U/mg) and its chromogenic substrate N-succinyl-AlaAla-Ala-P-nitroanilide (Thomson & Kapadia, 1979) that were acquired from the company Sigma Aldrich were used. The enzyme (at the final concentration of 10 Μ g/ml) was incubated in sodium phosphate buffer (50 mM, pH = 8) with the plant extracts at different concentrations (range from 1 to 20 Μ g/ml for aqueous extract and variation from 1 to 8 Μ g/ml for ethanolic extract) at 25 º C for A total final volume of 990 Μ L. After 30 minutes, 10 Μ L of substrate for a final concentration of 10 Μ M was added to the mixture and the hydrolysis of the chromogenic substrate was monitored for 5 minutes using a V-M5 Bel Photonics spectrophotometer at a wavelength of 410 nm. As negative control, phosphate buffer 50 mM, pH 8, and as positive control Epigalocatequina-3-galate were used.
All experiments were performed in triplicate and the data were presented as mean ± standard deviation. The values of the inhibition parameter IC50, which is the concentration of extract that inhibits 50% of the enzymatic activity, were determined by the percentage of remaining inhibition versus the extract concentration and calculated by non-linear regression using the GraFit 5.0 Program (Leatherbarrow, 1992). The percentage of inhibition was calculated by the equation:
% inhibition = | ABS Control – Abs Extract | /Abs control x 100%
RESULTS DISCUSSIONS
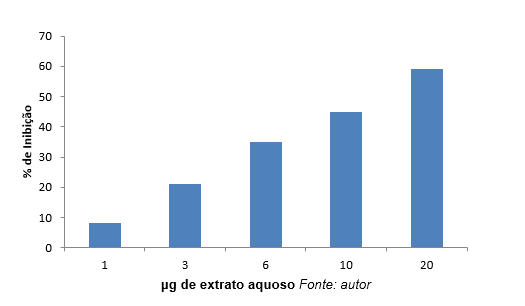
Graphs 1 and 2 show the percentage of inhibition due to different concentrations of aqueous and alcoholic extracts. These extracts inhibited elastase with different intensities. As shown in graph 1, the highest inhibition (59%) was observed in the concentration of 20 μg/ml of aqueous extract of the enzyme.
Graph 1: percentage (%) Inhibition of elastase as a function of different concentrations of aqueous extracts.
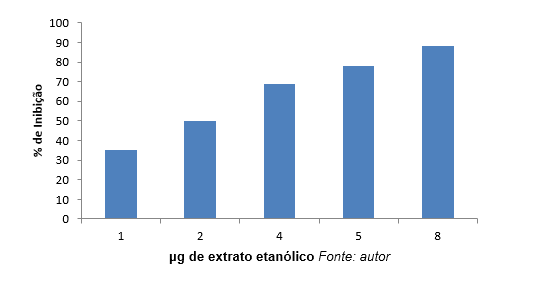
In graph 2, we can observe that the highest inhibition (88%) Concentration was 8 μg/ml.
Graph 2: percentage (%) Inhibition of elastase as a function of the concentration of ethanolic extracts.
From the results generated by graphs 1 and 2, the values of IC50 (table 1) were calculated for the two extracts, 8.1 ± 1.4 μg/ml (aqueous extract) and 3.31 ± 0.6 μg/ml (ethanolic extract). These results indicate that the ethanolic extract presented better inhibition power over the elastase than the aqueous extract.
Table 1: IC50 values of aqueous and ethanolic extracts on elastase. Values expressed as mean ± standard deviation.
| IC 50 (μg/ml) | |
| Elastase | |
| Aqueous extract | 8.1 ± 1.4 |
| Ethanolic Extract | 3.3 ± 0.6 |
Source: Author
Plant extracts provide limitless opportunities for discovering new enzyme inhibitors, given that they produce a wide variety of secondary metabolites. These chemical compounds are used by plants to protect themselves against the action of herbivorous animals and insects and many of these compounds are inhibitors of several classes of proteases, including Elastase (Sin & Kim, 2005; Middleton et al., 2000).
As previously described, the species Sedum Dendroideum produces a wide variety of secondary metabolites and these compounds may be involved in the inhibition of elastase.
Conclusions
The results of the present study indicated that the aqueous and ethanolic extracts of Sedum Dendroideum had a significant inhibitory activity on the proteolytic activity of elastase. More specific studies are needed to identify and isolate bioactive compounds in the extracts of Sedum dendroideum.
BIBLIOGRAPHICAL REFERENCES
ABBOUD, R.T.; VIMALANATHAN S. Pathogenesis of COPD. Part I: The role of protease-antiprotease imbalance in emphysema. International Journal of Tuberculosis and Lung Diseases. v.12, n.4, p.361–367, 2008.
AIMIN, H.; MINGSHI, W.; HONGYAN, H.; DECHENG, Z.; LEE, K. H. Hepatoprotective triterpenes from Sedum sarmentosum. Phytochemistry, Oxford, v. 49, n. 8, p. 2607-10, 1998.
COUTINHO, M. A. S., MUZITANO, M. F., COSTA, S. S., 2009. Flavonóides: Potenciais agentes terapêuticos para o processo inflamatório. Revista Virtual de Química 1, 241-256.
CUCCIOLONI, M., MOZZICAFREDDO, M., ONFILI, L., CECARINI, V., ELEUTERI, A.M., ANGELETTI, M., (2009): Natural occurring polyphenols as template for drug design. Focus on serine proteases. Chem Biol Drug Des., 74: 1–15.
EPAGRI − Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina. S. A. CD Plantas Medicinais. Versão 1.0. Itajaí, 1998. 1 CD-ROM.
HALIN, F.; SLOSSE, P.; HOOTELÉ, C. Sedum alkaloides – VII – Structure and synthesis of (+)-4- hydroxysedamine and (+)-4-hydroxyallosedamine. Tetrahedron, Oxford, v. 41, n. 14, p. 2891-7, 1985.
HART, H. T.; STEVENS, J. F.; JEONG, H. K. Alkaloids of some Asian Sedum species. Phytochemistry, Oxford, v. 41, n. 5, p. 1319-24, 1996.
HEIDARI-SURESHJANI M.; YAZDI F. T.; MORTAZAVI, S.A.; BEHBAHANI, B.A.; SHAHID F. Antimicrobial effects of Kelussia odoratissima extracts against food borne and food spoilage bacteria "in vitro". Journal of Paramedical Sciences, v. 5, n.2, 2015.
IBANEZ, S., GALLET, C., DESPRÉS, L., (2012): Plant Insecticidal Toxins in Ecological Networks. Toxins., 4: 228-243.
JOHANSSON, S. U., Neutrophil multitarget functional bioassay to detect anti-inflammatory natural products. J. Nat. Prod., v. 65, p. 32-41, 2002..
LEATHERBARROW, R.J. GraFit Version 5.0, Erithacus Software Ltd, Staines, U.K.,1992.
MIDDLETON, E., Kandaswami, C., Theoharides, T.C., (2000): The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease andCancer. Pharmacol Rev., 52:673-751.
MILANEZE, M. A.; GONÇALVES, E. Caracterização morfo-anatômica das folhas de Sedum dendroideum DC, CRASSULACEAE In: Simpósio Brasileiro De Farmacognosia, 3., 2001, Curitiba. Anais. Curitiba: Universidade Federal do Paraná, Departamento de Farmácia, Laboratório de Farmacognosia, 2001. p. FB-23.
OTÍN, C. L; BOND, J. S. Proteases: Multifunctional Enzymes in Life and Disease. Journal of Biological chemistry, v.283, 30433-30437, 2008.
RAWLINGS, N.D.; MORTON, F.R. The MEROPS batch BLAST: A tool to detect peptidases and their non-peptidase homologues in a genome. Biochimie, v. 90, p. 243-259, 2008.
SENDL, A.; MULINACCI, N.; VINCIERI, F. F.; WAGNER, H. Anti-inflammatory and immunologically active polysaccharides of Sedum telephium. Phytochemistry, Oxford, v. 34, n. 5, p. 1357-62, 1993.
SIEDLE, L.G., GUSTAVSSON, L., JOHANSSON, S., MURILLO, R., BOHLIN, L., The effect of sesquiterpene lactonas on the release of human neutrophil elastase. Biochem. Pharmacol, v. 7559, p. 1-7, 2003.
SIN, B.Y., KIM, H.P., (2005): Inhibition of collagenase by naturally-occurring flavonoids. Archives of Pharmacal Research., 28(10): 1152-1155.
STEVENS, J. F.; HART, H. T.; VAN HAM, R. C. H. J.; ELEMA, E. T.; VAN EN ENT, M. M.V.X.; WILDEBOER, M.; ZWAVING, J. H. Distribution of alkaloids and tannins in the Crassulaceae. Biochem. Syst. Ecol., Oxford, v. 23, n. 2, p. 157-65, 1995.
STUART, L.M; EZEKOWITZ, R.A. Phagocytosis: elegant complexity. Immunity, v. 5, p. 539-550, 2005.
THOMSON, A., KAPADIA, S.K., The Specificity of the S1 and S2 Subsites of Elastase, Eur.J.Biochem, v.102, p. 311-116, 1979.
[1] Graduate student in Biological sciences at IFSULDEMINAS-Campus Inconfidentes.
[2] PhD in biochemistry from UNIFESP; Effective Professor at IFSULDEMINAS-Campus Inconfidentes, and adjunct researcher to the Biochemistry Laboratory of the Environmental Procedures Center.
Submitted: May, 2019.
Approved: July, 2019.