ORIGINAL ARTICLE
SANTOS, Yasmim Barbosa dos [1], MELO, Ismaela Maria Ferreira de [2], ALVES, Érique Ricardo [3], NASCIMENTO, Bruno José do [4], SILVA, Maria Vanessa da [5], FRANÇA, Marcelle Mariana Sales de [6], PEREIRA, Alef de Moura [7], SOARES, Anísio Francisco [8], TEIXEIRA, Álvaro Aguiar Coelho [9], TEIXEIRA, Valéria Wanderley [10]
SANTOS, Yasmim Barbosa dos. et al. Melatonin and chronic ethanol consumption: effects on the offspring liver and kidney. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 08, Ed. 08, Vol. 04, pp. 133-151. August 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/biology/melatonin-and-chronic-ethanol, DOI: 10.32749/nucleodoconhecimento.com.br/biology/melatonin-and-chronic-ethanol
ABSTRACT
The aim of this research was to assess whether the administration of melatonin during pregnancy and lactation can mitigate alcohol-induced liver and kidney damage in the offspring. Three groups were formed with the offspring of 30 albino Wistar rats from the UFRPE bioterium: control group – Offspring of rats that did not consume alcohol; alcohol group – Offspring of rats that consumed alcohol; alcohol+mel group – Offspring of rats that consumed alcohol and received melatonin. The female rats received 3 g/Kg of alcohol and 0.8 mg/Kg of melatonin during the gestational and lactation period. The rat pups were euthanized at 30 days of age. The collected organs underwent histological procedures for morphometric, histopathological and statistical analyses. In the liver of the alcohol group, congestion in the portal and centrolobular veins, steatosis, and alterations in the sizes of hepatic parenchyma were observed, which were not present in the alcohol+mel and control groups. In the alcohol group, the kidneys also showed congestion in the cortical area, without the subcapsular space and with altered glomerulus size, on the other hand, the group treated with melatonin did not present alterations in these organs. The statistical analyses of the weights and lengths of the alcohol+mel group also did not reveal any significant alterations when compared to the group exposed only to alcohol. Thus, melatonin acted positively, interfering and mitigating the harmful effects that ethanol had on the liver, kidneys, weight and length of offspring whose mothers were subjected to chronic alcohol consumption.
Keywords: Alcohol, Antioxidant, Fetus, Free radicals, Mouse.
INTRODUCTION
Nearly 10% of women worldwide consume alcohol during the gestational period (POPOVA et al., 2017). This prevalence in the Americas is 11.2% according to the Pan American Health Organization (PAHO, 2019). This ingestion of alcoholic beverages during pregnancy is considered even more serious, as it can lead to various adverse consequences not only for the mother, but also for the embryo or fetus. These harmful results are known as Fetal Alcohol Spectrum Disorders (FASD), that includes mental, physical, cognitive and behavioral changes (BERTRAND et al., 2005).
The problems caused to the offspring of alcoholic mothers are considered the most harmful resulting from alcoholism, since alcohol reaches the fetal tissues easily (MESQUITA; SEGRE, 2009). In addition, according to some authors, alcohol can also reach breast milk in large or small amounts (BURGOS et al., 2002).
During alcohol metabolism, free radicals are released that increase oxidative stress and cause changes in protein activity (GONÇALVES; PEREIRA, 2007). The liver is primarily responsible for metabolizing alcohol, with the magnitude of liver damage being determined by the amount and regularity of consumption, which can cause hepatitis, hepatic steatosis, fibrosis, cirrhosis and hepatocellular carcinoma (LIEBER, 2005; FONSECA; RODRIGUES, 2018).
According to Batista et al., (2010) constant alcohol consumption can also cause various effects on the kidneys and in large amounts can lead to acute tubular necrosis syndrome. Thus, the consumption of alcoholic beverages is related to the promotion of chronic renal failure, in addition to authors presenting the relationship between alcoholism and changes in renal physiology and morphology (THIEL et al., 1977).
In view of this, the use of antioxidants has been an alternative to compensate for the damage caused by oxidative stress in organs, such as melatonin, which in addition to reducing the formation of free radicals and stimulating the action of antioxidant enzymes, it also decreases hepatic oxidation, protects nuclear and mitochondrial DNAs and increases messenger RNA levels for antioxidant enzymes (GUERRERO et al., 2007; SOLÍS-HERRUZO; SOLÍS-MUÑOZ, 2009; SOUZA; MORAIS, 2016). During the gestational period, maternal melatonin reaches the fetus through the placenta, so it can perform its functions in the fetus or embryo and in the first weeks of life, this hormone can reach the newborn through breast milk (ALVES et al., 1998; THOMAS et al., 2002; CARPENTIERI et al., 2012; REITER et al., 2013).
Considering the harmful effects that drinking too much alcohol can cause to the organism, it is pertinent to investigate the possible protective action of melatonin on the damage caused by ethanol in the offspring of rats. Thus, this research aimed to evaluate whether exogenous melatonin administered during pregnancy and lactation can prevent the deleterious effects caused by alcohol in the liver and kidneys of rat offspring.
MATERIAL AND METHODS
This research was conducted at the Federal Rural University of Pernambuco (UFRPE) and was approved by the Institutional Ethics Committee under the registration nº 3325300821. 30 virgin albino female rats (Rattus norvegicus albinus), 90 days old, weighing approximately 250±30g, from the Wistar lineage, were acquired through the bioterium of the Department of Animal Morphology and Physiology (DMFA) and took part in this study. The animals were provided with food and water and were housed in cages with an ambient temperature of 22±1°C. The lighting was provided by 40W fluorescent lamps (Philips brand, daylight model), establishing a 12-hour photoperiod. The female rats underwent colpocytological exams to identify the estrous cycle and those that presented three consecutive regular estrous cycles were selected for the experiment. After mating and confirmation of pregnancy, they were divided into 3 experimental groups with 10 animals each, for future formation of the following groups with the offspring: Control group – Rat pups who did not receive alcohol during pregnancy and lactation, euthanized after 30 days of life; Alcohol group – Rats pups subjected to chronic alcohol consumption during pregnancy and lactation, euthanized after 30 days of life; Alcohol + mel group – Rats pups rats subjected to chronic alcohol consumption and simultaneously treated with melatonin during pregnancy and lactation, euthanized after 30 days of life.
MATING OF ANIMALS
The females in the experiments were mated at the beginning of the night (18:00h). The following day, colpocytological examinations were performed using the Shorr-Harris staining method, taking the presence of sperm as a parameter for mating confirmation.
ETHANOL ADMINISTRATION
A dosage of 3 g/Kg of ethyl alcohol was administered intragastrically in rats during pregnancy (VARLINSKAYA et al., 2001; ARAÚJO-FILHO et al., 2007; VEIGA et al., 2007; SCHEIDT et al., 2015; MARCO et al., 2017).
TREATMENT WITH MELATONIN
Melatonin, N-acetyl-5-methoxytryptamine (Sigma Chemical Co., St. Louis, USA) was administered in daily injections of 0.8 mg/Kg, throughout pregnancy. For this purpose, melatonin was dissolved in 0.2 mL of ethanol and diluted in 0.8 mL of 0.9% NaCl. Injections were applied intraperitoneally, always between 6:00 pm and 7:00 pm. This dose is comparable to the human dose (9 mg/kg), which was converted based on body surface area (MOUSTAFA et al., 1999; ABD-ALLAH et al., 2003). The control and alcohol groups received the hormone vehicle.
WEIGHT AND MEASUREMENT OF ANIMALS
The weights and lengths of the pups were recorded on the day of birth, using a precision scale and measuring tape. And the averages of the data obtained were used for the statistical analyses.
HISTOPATHOLOGY
To collect the liver and kidneys from the pups at 30 days of age, the animals were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (6.0 mg/kg) intramuscularly. Next, the abdominal cavity was opened to remove the organs. Twelve puppies were used per group, regardless of sex. The animals were euthanized using anesthesia deepening with ketamine hydrochloride (80mg/Kg) and xylazine (6.0 mg/Kg), associated with thiopental (100 mg/kg), intraperitoneally. Liver and kidney fragments were immersed in buffered formaldehyde, remaining there for 48 hours. Then, parts of these organs went through the process of dehydration and diaphanization to embedded them in paraffin. The sections were made on a Minot type microtome (Leica RM 2035) adjusted to 5 mm. When obtained, they were placed on slides previously greased with Mayer’s albumin and placed to dry for 24 hours at a temperature of 37°C in an oven. Finally, the sections were stained with hematoxylin – eosin (H.E.) and analyzed in an OLYMPUS BX-49 light microscope and photographed in an OLYMPUS BX-50 microscope.
MORPHOMETRIC ANALYSIS
LIVER
The morphometric study was carried out according to the methodology described by Engelman et al., (2001). The proportion between the non-lobular and lobular parenchyma of the liver of the pups at 30 days of the experimental groups was determined by stereological methods, using a grid with 100 test points, placed on the sections of the histological preparations stained with Mallory’s trichrome, as this facilitates visualization of portal spaces when using stereological methods. Five slides were used for counting, considering 10 fields visualized through the 40x objective, concluding 5.000 points per group.
KIDNEYS
For the morphometric analysis of the kidneys of the puppies at 30 of the experimental groups, five slides from each group were used and ten glomeruli were analyzed on each slide. Measurements were restricted to the glomeruli, which demonstrated, in a single cut, the vascular and urinary poles. This arrangement indicates the section coinciding with the equatorial region of the glomerulus. For measurements, randomly selected glomeruli were used. With the use of a Sony® Video camera, coupled to the Olympus® Bx50 microscope, the images were recorded and the morphometric analysis was performed using the Line Morphometry application, calibrated in micrometers, associated with the Optimas® 6.2 program for Windows. The glomerular area value was obtained by positioning the cursor in order to draw an external circular line, coinciding with the limits of the glomerular tuft. To measure Bowman’s capsule, the same methodology was adopted (AKAOKA et al., 1994). To calculate the glomerulus and Bowman’s capsule volume, were used the criteria described by Pagtalunan et al., (2000). For this estimate, the equation 4/3πr3 was used, intended to calculate the volume of the sphere, in which “r” represents the radius.
STATISTICAL ANALYSIS
For the comparison of the morphometric data, the Analysis of Variance was performed, when significant this was complemented by the Multiple Comparisons test of Tukey and Kramer. A significance level of 0.05 (P <0.05) was adopted.
RESULTS
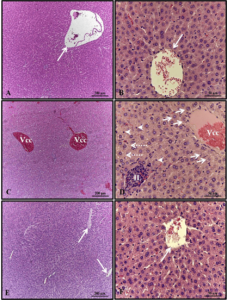
With regard to the weight and length of the pups after birth, there was a significant reduction in the group that received only alcohol (Figures 1 and 2). Histopathological analysis of the liver of the animals in the control group showed unchanged hepatic parenchyma with cords of organized hepatocytes bordering the central lobular vein, interspersed with sinusoidal capillaries. However, animals from born from rats that received alcohol during pregnancy and lactation showed hepatic parenchyma with portal and centrilobular vein congestion, in addition to a high degree of steatosis. These effects were not observed in the animals in the Alcohol + Mel group (Figure 3).
The kidneys of the animals in the control group were well preserved, with most of the glomeruli and subcapsular space well defined, in addition to proximal and distal convoluted tubules with normal characteristics, without any alteration. These characteristics were also verified in the kidneys of animals that received melatonin. In the kidneys of the animals that received only alcohol, the presence of areas of congestion in the cortex and corpuscles with absence of the subcapsular space were observed (Figure 4).
The morphometric analysis of the liver of animals, whose matrices received only alcohol during pregnancy and lactation, showed significantly reduced lobular parenchyma and increased non-lobular parenchyma. In the morphometric analysis of the kidneys, a significant reduction in the diameter and volume of the glomerulus, in addition to the diameter and volume of Bowman’s capsule, was also observed in this group. While the control and alcohol+mel groups did not present these alterations (Tables 1 and 2).
Figure 1: Weight of puppies at birth. Alcohol: Observe significant weight reduction; Control and Alcohol+Mel: There was no significant difference. Means followed by the same letter in the lines do not differ significantly by Tukey’s one-way Anova test with post hoc (p < 0.05 )
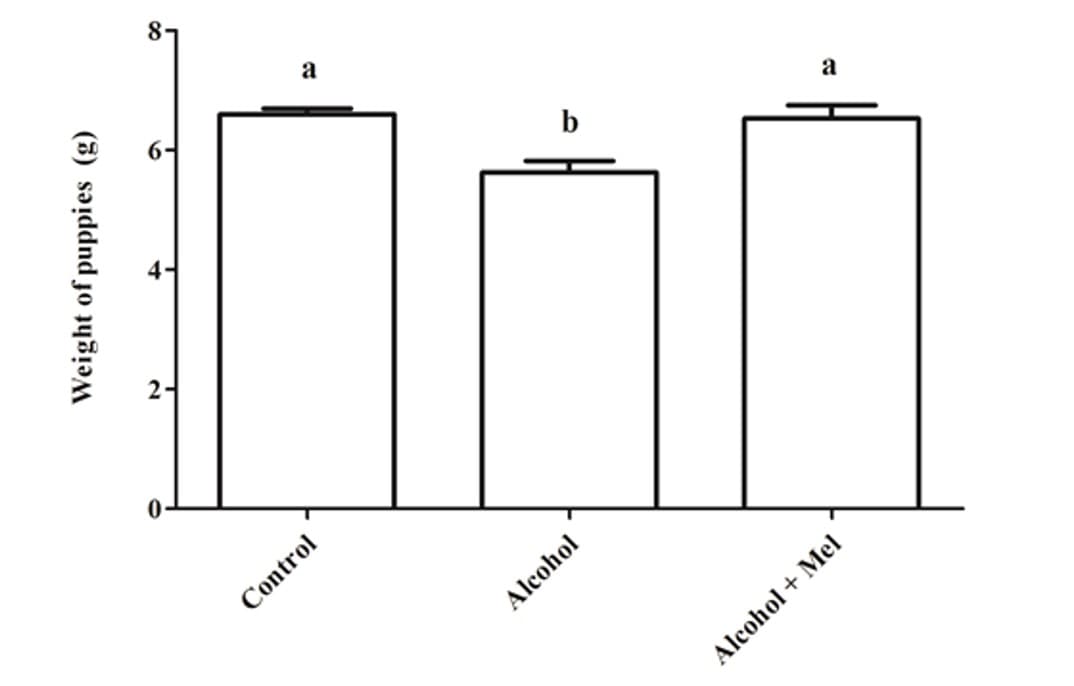
Figure 2: Length of pups at birth. Alcohol: Observe significant reduction in length; Control and Alcohol+Mel: There was no significant difference. Means followed by the same letter in the rows do not differ significantly by Tukey’s one-way Anova test with post hoc (p < 0.05)
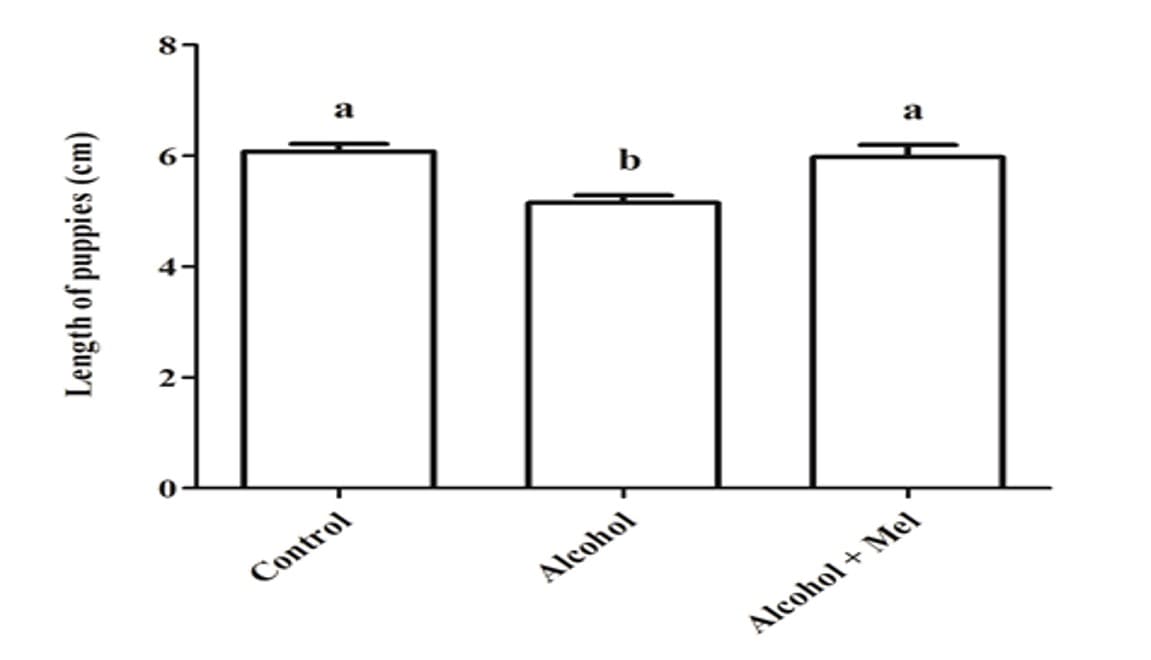
Figure 3: Photomicrograph of the animals’ liver at 30 days of age. (AB) – Control: without alterations with cords of organized hepatocytes; (CD) – Alcohol: observe portal and centrilobular vein congestion and high degree of steatosis; (EF) – Alcohol + Mel: structures similar to the control. Long arrows – centrilobular vein; Short arrows – cords of hepatocytes; Asterisk – biliferous duct; Arrowheads – sinusoids; Dashed arrows – co-management of the centrilobular vein; White arrows – hepatocytes showing steatosis. HE
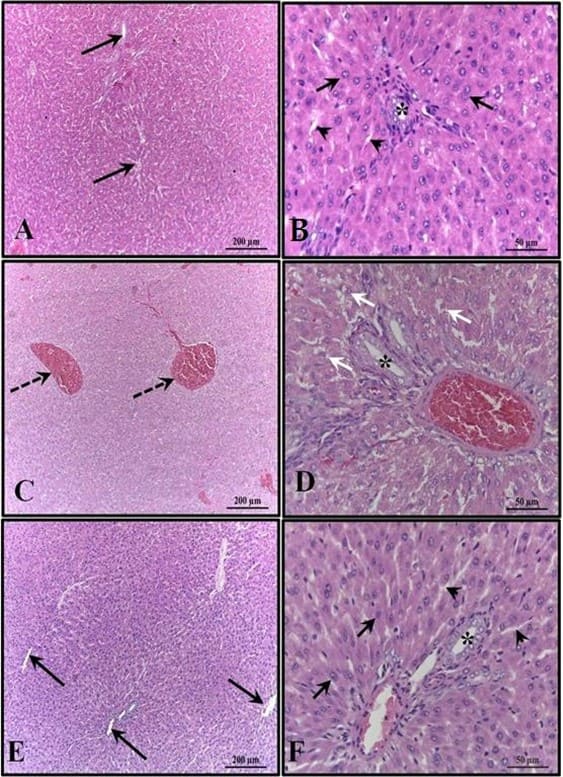
Figure 4: Photomicrograph of the animals’ kidneys at 30 days of age. (AB) – Control: preserved histological structure; (CD) – Alcohol: observe the presence of areas of congestion in the cortex and corpuscles with absence of the subcapsular space; (EF) – Alcohol + Mel: structures similar to the control. Long arrows – Bowman’s capsule; Asterisk – glomerulus; Dashed arrows – cortical congestion; Short arrows – absence of subcapsular space; Rc – Cortical Region. HE
Table 1. Mean ± standard deviation of the percentage of lobular and non-lobular parenchyma in the liver of animals at 30 days of life.
| groups | Control | Alcohol | alcohol + mel | P |
| Lobular Parenchyma | 81.59 ± 2.95y | 73.26 ± 1.40b | 80.53 ± 1.42a | 0.0050 |
| Non-Lobular Parenchyma | 18.43 ± 2.97y | 26.50 ± 1.04b | 19.50 ± 1.48y | 0.0053 |
Source: Authors, 2023.
Means followed by the same letter in the rows do not differ significantly by Tukey’s one-way Anova test with post hoc (p < 0.05).
Table 2. Mean ± standard deviation of glomerular diameter (DG), glomerulus volume (VG), diameter (DCB), volume (VCB) of Bowman’s capsule in the kidneys of animals at 30 days of life.
| groups | Control | Alcohol | alcohol + mel | P | |
| DG (µm) | 6629 ± 351a | 4670 ± 121b | 6302 ± 157a | 0.0017 | |
| VG (µm 3) | 35212 ± 3584a | 20329 ± 1734b | 33319 ± 216a | 0.0094 | |
| DCB(µm) | 7338 ± 296a | 5877 ± 380b | 7202 ± 110a | 0.0024 | |
| VCB(µm3) | 41645 ± 544a | 30881 ± 761b | 42972 ± 464a | 0.0317 | |
Source: Authors, 2023.
Tukey’s one-way Anova test with post hoc (p < 0.05).
DISCUSSION
Intrauterine exposure to alcohol can cause several deleterious effects to the fetus or embryo, such as structural anomalies and behavioral and neurocognitive deficiencies, and one of the first prenatal symptoms, growth deficiency (length and / or weight) that possibly persists in the postnatal period (HOYME et al., 2005).
In this work, a decrease in the weight and length of the offspring was observed with the consumption of alcohol by the sows, while in the control and alcohol+mel groups there were no significant differences for this result. Authors such as Domingues et al., (2009) also found the interference of ethanol in the growth of fetuses. Since excessive consumption of alcohol can impair the maternal ability to maintain the development of the fetus, interfering with the absorption of nutrients by the mother and causing vasoconstriction of the umbilical cord and placenta, altering its ability to provide nutrients to the fetus through the flow blood (GOODLETT; HORN, 2001).
In turn, melatonin in the alcohol+mel group was able to preserve fetal growth, since it has an antioxidant action, protecting the placenta against damage, in addition to stimulating the passage of nutrients via the umbilical cord (EIFERT et al., 2015). As well as, Nagai et al., (2008) found in their study that treatment with melatonin prevented fetal growth restriction and oxidative damage to the placenta.
In the histopathological and morphometric analyzes of the liver in the alcohol group, congestion in the centrilobular and portal veins was detected, in addition to steatosis, which is considered the first stage of alcoholic liver disease, being a direct consequence of the impacts caused by alcohol metabolism (BREITKOPF et al., 2009). Steatosis can be caused by the accumulation of NADH in hepatocytes during ethanol metabolism, causing changes in lipid metabolism by inhibiting the β-oxidation of fatty acids and increasing their synthesis to dispose of excess hydrogen (ALBANO, 2006; SOZIO; CRABB, 2008).
On the other hand, these alterations were not observed in the alcohol+mel group, indicating the beneficial action of melatonin and demonstrating its efficacy in protecting the offspring’s liver from injuries resulting from alcohol exposure. The antioxidant and anti-inflammatory activities of melatonin are well-known, in addition to its protective properties against oxidative stress and its ability to prevent alcoholic hepatic steatosis (ZHANG et al., 2017). According to Hu et al., (2009) melatonin demonstrated the ability to inhibit the generation of free radicals, resulting in a considerable reduction in steatosis, lipid peroxidation and inflammatory cytokine activity in adult rats exposed to alcohol consumption. Our work showed that the administration of melatonin can also stop the development of liver damage caused in the offspring of mothers who consume alcohol during pregnancy and lactation.
In the alcohol group, histological and morphometric findings also showed congestion in the cortical region and absence of subcapsular space in the renal corpuscles, in addition to a significant reduction in the volume and diameter of the glomeruli and Bowman’s capsule. Studies have already confirmed the relationship between Atherosclerotic Renal Artery Disease (ARAD) and chronic alcohol consumption, which leads to kidney atrophy, as well as the increased production of reactive oxygen species by ethanol metabolism, which can affect the kidneys and damage the renal tubules, these free radicals also affect cells of the immune system and stimulate the production of pro-inflammatory cytokines, which can lead to the development of kidney diseases (KONOPKA et al., 2007; BARR et al., 2016; VARGA et al., 2017; WANG et al., 2017).
The alcohol + mel group did not reveal these lesions in the kidneys, which indicates the action of melatonin with its anti-inflammatory, antioxidant and cytokine modulating effects on this organ as well. Treatment with melatonin is capable of significantly reducing oxidative stress on the kidneys, in addition to also exerting an anti-apoptotic action, with the ability to reduce pro-inflammatory cytokines, improve mitochondrial activity and have beneficial effects on changes in renal pressure (RUSSCHER et al., 2012, ZHANG et al., 2021). This explains the protection against changes in renal histology and how it avoided congestion caused by the stimulation of inflammatory actions in the alcohol + mel group, since melatonin has a range of biological activities performed in different organs through its receptors. Studies with adult rats show that melatonin prevents the structural and functional impairment of renal tissue caused by ethanol-induced oxidative stress (KURHALUK et al., 2020). However, our findings indicate that melatonin is able to attenuate these lesions also in pups that were exposed to ethanol during pregnancy and lactation.
CONCLUSIONS
Thus, melatonin was able to interfere and mitigate the harmful effects that ethanol exerted on the histology of the liver and kidneys of offspring whose sows were subjected to chronic alcohol consumption. This research also showed a positive effect of this hormone on weight and length, meaning that excess alcohol does not alter the absorption and transport of nutrients between mother and offspring. Thus, suggesting that melatonin may be an important adjuvant in the treatment of damage caused by alcohol to the liver and kidneys of children of alcoholic women.
REFERENCES
ALBANO, E. Alcohol, oxidative stress and free radical damage. Proceed. of the Nutri. Society., v.65, n.3, p. 278-290, 2006.
ABD-ALLAH, A. R. A. et al. Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol. Res., v.47, n.4, p.349 – 354, 2003.
AKAOKA, K.; WHITE, R. H.; RAAFAT, F. Human glomerular growth during childhood: a morphometric study. J. Pathol., v.173, n.3, p.261-268, 1994.
ALVES, R. S. C. et al. Melatonin and sleep in children. Div. Ped., v.20, n.2, p.99- 954, 1998.
ARAÚJO-FILHO, J. L. S. et al. Histomorphometric analysis of the heart of rats indirectly exposed to ethanol and chronic malnutrition during the perinatal period. Rev. Ciênc. Méd. Biol., v.6, n.1, p.17-25, 2007.
BARR, T. et al. Opposing effects of alcohol on the immune system. Prog Neuropsychopharmacol Biol Psychiatry., v. 65, p. 242-251, 2016.
BATISTA, A. H.; SEGUI, G. P.; CANDINA, H. R. Alteraciones en las características morfométricas del riñón de ratas albinas machos provocadas por la ingestión crónica de etanol desde la adolescencia. Rev. Cubana Invest. Biomédicas, v. 29, n. 2, p. 194-202, 2010.
BERTRAND, J.; FLOYD, R. L.; WEBER, M. K. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep., v. 54, n.11, p. 1-12, 2005.
BREITKOPF, K. et al. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res., v.33, n.10, p. 1647-1655, 2009.
BURGOS, M. G. P. A. et al. The effect of alcoholic beverages in nursing mothers and their impact on children. Rev. Bras. Saúde Matern. Infant., v.2, n. 2, p.129-135, 2002.
CARPENTIERI, A. et al. New perspectives in melatonin uses. Pharmacol. Research., v. 65, n.4, p. 437-444, 2012.
DOMINGUES, J. A. et al. Maternal and fetal liver histomorphological analysis of malnourished pregnant rats submitted to exposure to ethanol. Rev. da Facul. de Ciências Méd. de Sorocaba, v. 11, n. 3, p.9-17, 2009.
EIFERT, A. W. et al. Effect of melatonin or maternal nutrient restriction on vascularity and cell proliferation in the ovine placenta. Anim Reprod Sci., v.153, p. 13-21, 2015.
ENGELMAN, M. F. B. et al. Morphometric study of the liver of rats submitted to supraphysiological doses of thyroxine. Arq. Bras. Endocrinol. Metab., v.45, n.2, p.173-179, 2001.
FONSECA, C. F.; RODRIGUES, F. F. Action of ethanol on the liver. Altus Ciência: Rev. Acad. Multi. da Facul. Cid. de João Pinheiro. v.7, n.7, p. 75-90, 2018.
GONÇALVES, C. S.; PEREIRA, F. E. L. Alcoholic Hepatopathy: Pathogenesis and Treatment. Prog. de Educação Médica Continuada: Socied. Bras. De Hepatol., v.7, n.102, p. 1799-1807, 2007.
GOODLETT, C. R.; HORN, K. H. Mechanisms of Alcohol-Induced Damage to the Developing Nervous System. National Institute on Alcohol Abuse and Alcoholism, v.25, n.3, p.175-184, 2001.
GUERRERO, J. M. et al. La Melatonina. Investig. y Cienc., p.30-38, 2007.
HOYME, H. E. et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics, v.115, n.1, p. 39-47, 2005.
HU, S. et al. Melatonin protects against acoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Europ. J. of Pharmacol., v.616, n.1-3, p. 287-292, 2009.
KONOPKA, C. L. et al. Experimental model for the study of chronic renal ischemia in rats: morphologic, histological and ultra-structural analysis. Acta Cir Bras., v.22, n. 1, p.12-21, 2007.
KURHALUK, N. et al. Melatonin modulates oxidative phosphorylation, hepatic and kidney autophagy-caused subclinical endotoxemia and acute ethanol-induced oxidative stress. Chronobiol Int., v. 37, n. 12, p. 1709-1724, 2020.
LIEBER, C. S. Metabolism of Alcohol. Clinics in Liver Disease, v.9, n.1, p. 1–35, 2005.
MARCO, E. M. et al. Long-term effects of intermittent adolescent alcohol exposure in male and female rats. Front. Behav. Neurosci., v.11, n.233, p.1-13, 2017.
MESQUITA, M. A.; SEGRE, C. A. M. Frequency of alcohol effects in fetus and pattern of alcohol consumption by pregnant women at a public maternity hospital in São Paulo city, Brazil. Rev Bras Crescimento Desenvol. Hum., v.1, n.19, p. 63-77, 2009.
MOUSTAFA, A. M. et al. Effect of bromocriptine on uterine contractility in near term pregnant rats. Pharmacol. Res., v. 39, n.2, p. 89 – 95, 1999.
NAGAI, R. et al. Melatonin Preserves Fetal Growth in Rats by Protecting against Ischemia/Reperfusion-Induced Oxidative/Nitrosative Mitochondrial Damage in the Placenta. J. of Pineal Research., v. 45, n. 3, p. 271– 76, 2008.
PAHO (Pan American Health Organization). PAHO virtual course addresses alcohol consumption during pregnancy, 2019. Disponível em: https://www.paho.org/pt/noticias/25-6-2019-curso-virtual-da-opas-aborda-consumo-alcool-durante-gravidez
PAGTALUNAN, M. E. et al. Methods for estimating the volume of individual glomeruli. Kidney Int., v. 57, p. 2644-2649, 2000.
POPOVA, S. et al. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob. Health, v. 5, p. 290-299, 2017.
REITER, R. J. et al. Melatonin and stable ccircadian rhythms optimize maternal, placental and fetal physiology. Human Reprod. Update, v.20, n.2, p. 293–307, 2013.
RUSSCHER, M. et al. The role of melatonin treatment in chronic kidney disease. Front Biosci., v.17, n. 7, p. 2644-56, 2012.
SCHEIDT, L. et al. Ethanol during adolescence decreased the BDNF levels in the hippocampus in adult male Wistar rats, but did not alter aggressive and anxiety-like behaviors. Trends Psych. Psychother., v.7, n.3, p. 143-151, 2015.
SOLÍS-HERRUZO, J. A.; SOLÍS-MUÑOZ, P. Melatonin and oxidative stress. Rev. Esp. de Enfermeiras Digest., v.101, n. 7, p. 453- 459, 2009.
SOUZA, W. L.; MORAIS, E. A. Antioxidant activity of melatonin on oxidative stress in spermatozoa: literature review. Nutri. Rev. Eletrônica, v.13, n. 5, p.4831-4839, 2016.
SOZIO, M.; CRABB, D. W. Alcohol and lipid metabolism. J. americ. de fisiol. Endocrin. e metabol., v.295, n. 1, 2008.
THOMAS, L. et al. Melatonin receptors in human fetal brain: 2- [125I] iodomelatonin binding and MT1 gene expression. J. of Pineal Research, v. 33, n. 4, p.218–224, 2002.
THIEL, D. H. et al. Alcohol: Its effect on the kidney. Metabolism, v. 26, n. 8, p. 857–866, 1977.
VARGA, Z. V. et al. Alcohol misuse and kidney injury: Epidemiological evidence and potential mechanisms. Alcoh. research: current reviews, v.38, n. 2, p. 283-288, 2017.
VARLINSKAYA, E. I. et al. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clin. Exp. Res., v.25, n.3, p. 377–385, 2001.
VEIGA, R. K. A. et al. Morphometric changes in the thymus, spleen and Peyer’s patches during pre and postnatal alcohol exposure. Rev. Eletronica Farm., v.4, n.1, p. 32-42, 2007.
WANG, L. et al. Effects of chronic alcohol exposure on ischemia-reperfusion-induce acute kidney injury in mice: the role of β-arrestin 2 and glycogen synthase kinase. Exper. & Mol. Medic., v. 49, n. 6, 2017.
ZHANG, C. et al. Melatonin Alleviates Contrast-Induced Acute Kidney Injury by Activation of Sirt3. Oxid Med Cell Longev., v. 25, 2021.
ZHANG, J. J. et al. Effects of melatonin on liver injuries and diseases. Inter. J. of mol. Sci., v. 18, n. 4, p. 673, 2017.
[1] Master by the Graduate Program in Animal Bioscience -UFRPE. ORCID: 0000-0002-6228-6951. Curriculum Lattes: http://lattes.cnpq.br/1783975917572458.
[2] PhD by the Graduate Program in Animal Bioscience/UFRPE. ORCID: 0000-0002-4150-1923. Lattes curriculum: https://lattes.cnpq.br/3537458174521270.
[3] Doctor by the Graduate Program in Animal Bioscience. ORCID: 0000-0002-7925-9212. Curriculum Lattes: http://lattes.cnpq.br/6892417222004207.
[4] Master by the Graduate Program in Animal Bioscience. ORCID: 0000-0001-9404-7501. Curriculum Lattes: http://lattes.cnpq.br/8213260513385508.
[5] Master by the Graduate Program in Animal Bioscience. ORCID: 0000-0002-4733-461X. Curriculum LatteS: http://lattes.cnpq.br/1906334502843226.
[6] Bachelor’s Degree in Biological Sciences. ORCID: 0000-0002-9662-0372. CURRICULUM LATTES: http://lattes.cnpq.br/2810228771568915.
[7] Bachelor’s Degree in Biological Sciences – UFRPE. ORCID: 0000-0003-3659-3947. Curriculum Lattes: http://lattes.cnpq.br/5069796237775832.
[8] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: 0000-0003-1493-7964. Curriculum Lattes: http://lattes.cnpq.br/9044747136928972.
[9] PhD in Morphology/UNIFESP-EPM. ORCID: 0000-0001-5940-9220. Curriculum Lattes: http://lattes.cnpq.br/1539131079574469.
[10] Advisor. PhD in Nuclear Technology from the University of São Paulo (USP). ORCID: 0000-0001-9533-5476. Curriculum Lattes: http://lattes.cnpq.br/4292195468804301.
Submitted: July 11, 2023.
Approved: August 08, 2023.