ORIGINAL ARTICLE
NASCIMENTO, Bruno José do [1], PEREIRA, Alef de Moura [2], BRAGA, Valeska Andrea Ático [3], SILVA, Maria Vanessa da [4], SANTOS, Yasmim Barbosa dos [5], LAPA NETO, Clovis José Cavalcanti [6], MEDINA, Vanessa Bischoff [7], SOARES, Anísio Francisco [8], TEIXEIRA, Álvaro Aguiar Coelho [9], TEIXEIRA, Valéria Wanderley [10]
NASCIMENTO, Bruno José do. et al. Effect of melatonin on the liver histophysiology of adolescent rats exposed to alcohol. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 08, Ed. 08, Vol. 04, pp. 152-174. August 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/biology/histophysiology-of-adolescent-rats, DOI: 10.32749/nucleodoconhecimento.com.br/biology/histophysiology-of-adolescent-rats
ABSTRACT
The present study evaluated whether melatonin administered during adolescence can prevent the deleterious effects produced by alcohol on the liver. Thirty albino female rats (Rattus norvegicus albinus), 40 days old, virgins, weighing approximately 150±10g, from the Wistar lineage and were divided into the following groups: I – Adolescent rats that did not receive alcohol and euthanized at 60 days of life; II – Adolescent rats subjected to chronic alcohol consumption and euthanized at 60 days of life; III – Adolescent rats subjected to chronic alcohol consumption and simultaneously treated with melatonin, and euthanized at 60 days of life. The collected organs underwent histological processing and to obtain the results, morphometric, histopathological and histochemical analyzes were performed. Alcohol was administered by gavage. intragastric injection at a dosage of 3 g/Kg of ethyl alcohol in the rats of groups II and III for 20 days. Melatonin was administered in daily injections of 0.8 mg/Kg, always at the beginning of the night, intraperitoneally for 20 days. When analyzing the weight of the animals, no significant difference was observed between the groups. The animals in group II presented alterations in the livers such as congestion of the centrilobular vein, hepatocellular ballooning, microgoticular steatosis, leukocyte infiltrate, several pyknotic nuclei in the livers, increase in lobular parenchyma and reduction in non-lobular parenchyma, greater deposition of collagen and reduction of glycogen. Melatonin treatment prevented all these changes. With this, we can conclude that melatonin has great therapeutic potential in the prevention of liver damage in adolescent rats subjected to moderate alcohol consumption, in addition to positive effects on the deposition of collagen and glycogen in the liver.
Keywords: Teenagers, Alcohol, Antioxidant, Liver, Inflammation.
1. INTRODUCTION
The consumption of alcoholic beverages is common worldwide, and factors such as age, culture, gender and religion influence both the frequency and the volume consumed. About 43% of the global population consumes alcoholic beverages, in the Americas region, this index is higher than the global one, reaching 54% (WHO, 2018). In Brazil, approximately 25 million inhabitants, which is equivalent to 16.5% of the population, consumed more alcohol in 2015 (FIOCRUZ, 2017). According to Andrade; Siu (2019), in 2016, the Americas region was the third in the world in relation to excessive alcohol consumption by young people, registering a percentage of 18.5%, behind only the West Pacific (18.8%) and Europe (24.1%). In Brazil, young people aged 18 to 24 years are in second place in the proportion of alcohol consumption, corresponding to 35.1%, second only to individuals aged 35 to 34 years (38.1%) (FIOCRUZ, 2017). According to WHO (2018), in 2016, the prevalence of women who consumed alcohol was 32.3%, lower than that of men, which was 53.6%. Despite the indices pointing to lower consumption of alcoholic beverages by women, they are more affected, as they are biologically more vulnerable to the effects of alcohol than men. Thus, they are more likely to develop alcohol-related problems even with lower consumption levels and/or at an earlier age than men (ANDRADE; SIU, 2019).
EtOH is a small molecule, uncharged and soluble in water, and for this reason it can easily cross cell membranes (NORBERG et al., 2003; VONGHIA et al., 2008). Ingested alcohol is metabolized by the liver, where the largest amount of metabolizing enzymes is present (MINCIS; et al, 2011). After its absorption, EtOH is converted by Alcohol Dehydrogenase (ADH), catalase or cytochrome P450 2E1 (CYP2E1) into acetaldehyde. After this step, Aldehyde Dehydrogenase (ALDH) converts acetaldehyde into acetate (BEST; LAPOSATA, 2003). These reactions promote increased respiratory chain activity, and consequently increased ROS production (BROCARDO et al., 2011). Alcohol products have been considered hepatotoxins that act directly or indirectly on the organ (ROCCO et al., 2014), in addition to causing necrosis or apoptosis of hepatocytes (FARKAS; KEMÉNY, 2013).
As the liver is the main organ responsible for metabolizing alcohol, it is the one that suffers most from the harmful effects of this xenobiotic (ROCCO et al., 2014). Continuous exposure to EtOH leads to the progression of fat accumulation in the liver, steatosis, to the inflamed state, steatohepatitis (YANG et al., 2010). Steatosis is the initial alcoholic disease, later subsequent inflammation and alcoholic hepatitis may occur, resulting in liver fibrosis (FARKAS; KEMÉNY, 2013).
Melatonin, a hormone produced mainly by the pineal gland, with the ability to perform numerous receptor-dependent and independent mediated actions, may act in the control of the circadian cycle, promoting sleep and performing an antioxidant action, reducing oxidative stress, in addition to having anti- inflammatory and anti- apoptotic drugs (HARDELAND; PANDI-PERUMAL; CARDINALI, 2006; MAHIEU et al., 2009; REITER; TAN; GALANO, 2014). Its antioxidant capacity was discovered in 1993 (POEGGELER et al., 1993), and has been used in the prevention of diseases caused by oxidative stress, due to its ability to eliminate free radicals and improve antioxidant levels (NWOZO; AJAGBE; OYINLOYE, 2012). This indolamine and its metabolites act in the direct elimination of several free radicals, such as the hydroxyl radical (OH), peroxynitrite (ONOO), nitric oxide (NO) and O2 radical, in addition to the reactive species of oxygen, nitrogen and sulfur (AMARAL et al., 2019; GALANO; TAN; REITER et al., 2018; GUNATA; PARLAKPINAR; ACET, 2020).
With regard to the liver, some studies with rats subjected to alcohol consumption have already demonstrated positive aspects of melatonin administration on the liver structure (KURCER et al., 2007; KURHALUK; TKACHENKO; LUKASH, 2020), on the activity of antioxidant enzymes (KURCER et al., 2007), inhibition of pro-inflammatory cytokines (HU et al., 2019) and apoptosis (ULLAH et al., 2020). Thus, this indolamine has great potential to prevent changes caused by alcohol. With this, this work aimed to evaluate whether melatonin administered to adolescent rats could prevent the deleterious effects produced by alcohol in the liver.
2. MATERIAL AND METHODS
2.1 ANIMAL ACQUISITION
Thirty albino female rats (Rattus norvegicus albinus), 40 days old, virgins, weighing approximately 130±10g, of the Wistar lineage, coming from the Vivarium of the Department of Animal Morphology and Physiology (DMFA), of the Federal Rural University of Pernambuco (UFRPE) were used. The animals were confined in cages and maintained with food and water ad libitum, with a temperature of 22±1ºC and artificial lighting produced by fluorescent lamps (Phillips brand, daylight model, 40W), with a 12-hour photoperiod. light and dark for 12 hours, considering the light period from 6:00 am to 6:00 pm. After an adaptation period of seven days, vaginal smears were taken to determine the estrous cycle. Females that had three consecutive regular estrous cycles were selected for the experiment, which consisted of the following experimental groups with 10 animals each:
Control – Adolescent rats that did not receive alcohol and euthanized at 60 days of age;
Alcohol – Adolescent rats subjected to chronic alcohol consumption and euthanized at 60 days of life;
Alcohol + Melatonin – Adolescent rats subjected to chronic alcohol consumption and simultaneously treated with melatonin and euthanized at 60 days of life.
The experiments were approved by the institutional ethics committee under license CEUA/UFRPE Nº 041/2019.
2.2 ETHANOL ADMINISTRATION
It was administered by gavage intragastric injection at a dosage of 3 g/kg of ethyl alcohol in the rats of groups II and III for 20 days (ARAÚJO-FILHO et al., 2007; MARCO et al., 2017; SCHEIDT et al., 2015; VARLINSKAYA; SPEAR; SPEAR, 2001; VEIGA et al., 2007).
2.3 MELATONIN TREATMENT
Melatonin, N-acetyl-5-methoxytryptamine (Sigma Chemical Co., St. Louis, USA) was administered in daily injections of 0.8 mg/kg, for a period of 20 days. For this, melatonin was dissolved in 0.2 mL of ethanol and diluted in 0.8 mL 0.9% NaCl. Injections were applied intraperitoneally, between 6:00 pm and 7:00 pm. This dose is comparable to the human dose (9 mg/kg), which was converted based on body surface area (ABD-ALLAH et al., 2003; MOUSTAFA et al., 1999; PAGET; BARNE, 1994). The animals in groups I and II received the hormone vehicle.
2.4 WEIGHT OF ANIMALS
The rats were weighed daily throughout the experimental period and for statistical analysis days 1, 10 and 20 were considered.
2.5 HISTOPATHOLOGY AND HISTOCHEMISTRY OF THE LIVER
For liver collection, the rats were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (6.0 mg/kg) intramuscularly. Then, the abdominal cavity was opened to remove the organ. The rats were euthanized using anesthesia deepening with ketamine hydrochloride (80mg/Kg) and xylazine (6mg/Kg), associated with thiopental (100 mg/kg), intraperitoneally. Liver fragments were immersed in buffered formalin at 10% and pH 7.4, remaining there for 48 hours. After these procedures, they were dehydrated in ethyl alcohol (increasing concentrations), cleared with xylol, impregnated and included in paraffin. The paraffin blocks were cut in a Minot type microtome (Leica RM 2035) adjusted to 5 μm. The sections obtained in this way were placed on glass slides and taken to an oven regulated at a temperature of 37°C for 24 hours, for drying and bonding. In sequence, the sections were stained with hematoxylin-eosin (HE), picrosirius and PAS (Periodic Acid Schiff) and analyzed in an OLYMPUS BX-49 light microscope and photographed in an OLYMPUS BX-50 microscope.
2.6 MORPHOMETRY
Morphometric study was carried out according to the methodology described by Engelman et al. (2001). The proportion between the non-lobular and lobular parenchyma of the liver of the rats in the experimental groups was determined by stereological methods, using a grid with 100 test points, placed on the sections of the histological preparations stained with Mallory’s trichrome, as this facilitates visualization. of the portal spaces when using stereological methods. The counting was done on three slides, so that 10 fields were counted using the 40x objective, making a total of 3,000 points per group.
A semi-quantitative assessment of liver change scores proposed by the NASH Committee on Pathology (Nonalcoholic Steatohepatitis) Clinical Research Network (KLEINER et al., 2005), which are required to follow: steatosis (<5% = 0; 5– 33% = 1; 33–66% = 2; > 66% = 3); lobular inflammation (none = 0; 2 foci = 1; 2-4 foci = 2; > 4 foci = 3); and hepatocellular ballooning (none = 0; few = 1; prominent = 2). All features were blindly scored on three slides/group, evaluated five fields/slide. For this, the images were captured with a Sony® video camera coupled to an Olympus® Bx50 microscope. All quantifications were performed using the Gimp®2.8 image editor.
2.7 STATISTICAL ANALYSIS
To compare the weight of the rats, morphometry and triage and histochemistry data, the Analysis of Variance was performed, when significant, it was complemented by the Multiple Comparisons test by Tukey and Kramer. A significance level of 0.05 (P < 0.05) was adopted.
3. RESULTS
3.1 WEIGHT OF ANIMALS
There was no significant difference between the weights of rats treated with alcohol, alcohol + melatonin when compared to the control group on days 1, 10 and 20 (Table 1).
Table 1: Mean ± standard deviation of the weight of the rats in the experimental groups, on days 1, 10 and 20
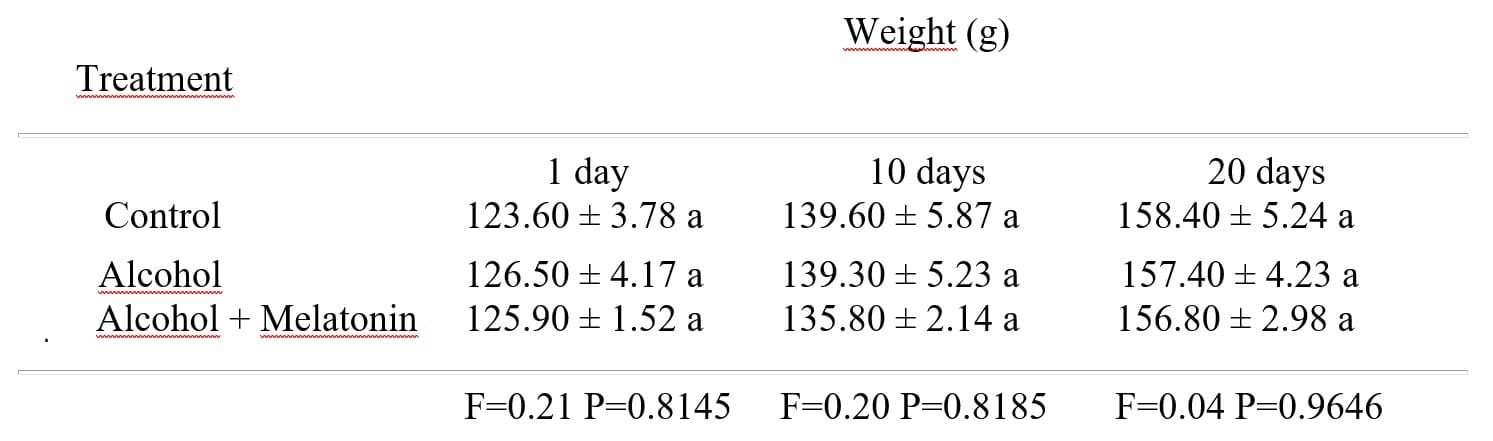
1 Means followed by the same letter do not differ according to the Tukey and Kramer test (P<0.05).
3.2 HISTOPATHOLOGICAL ANALYSIS OF THE LIVER
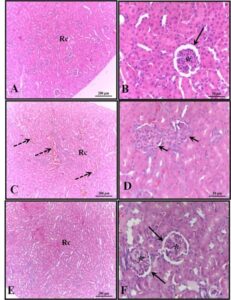
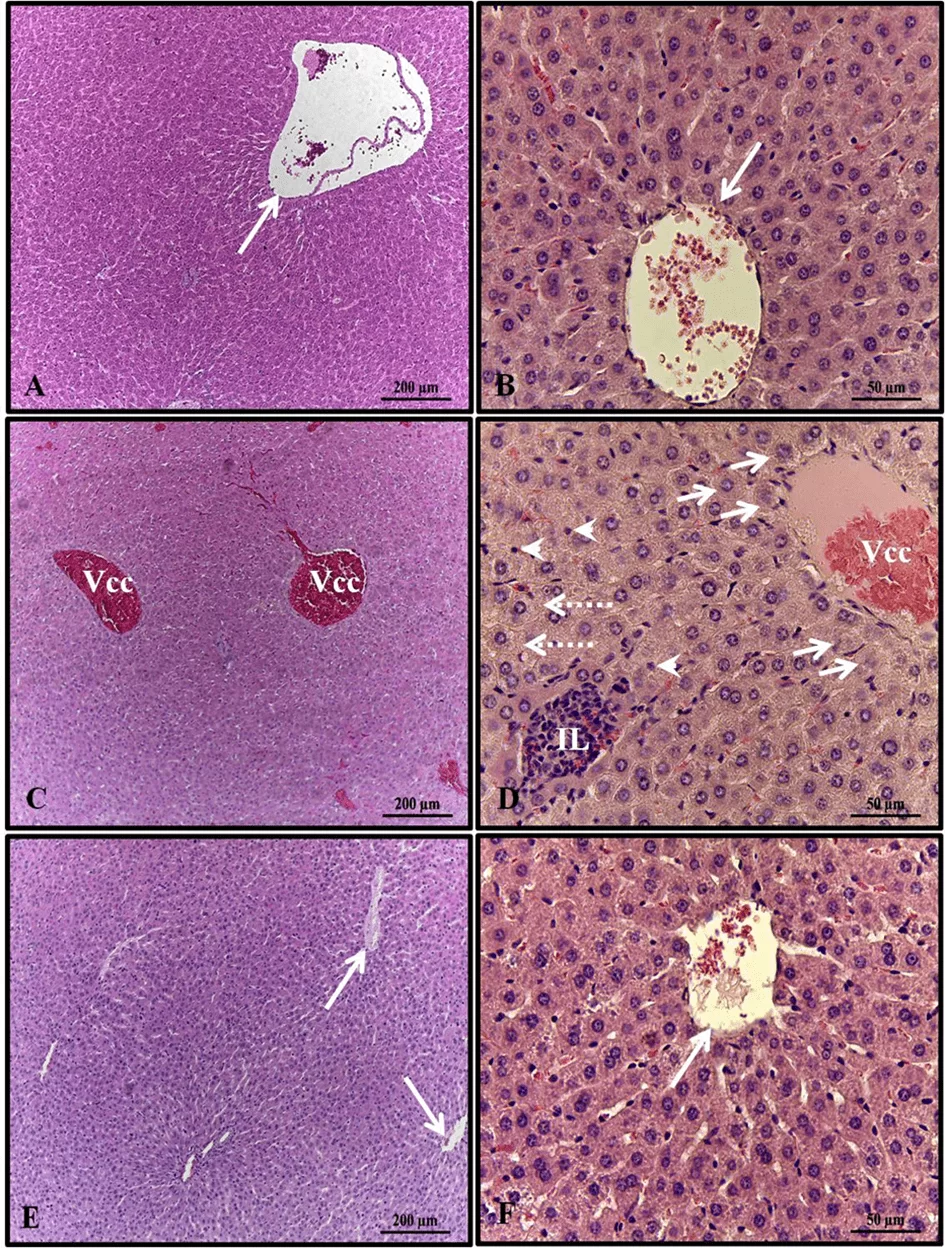
Histopathological analysis of the liver of females in the control and alcohol + melatonin groups revealed unchanged hepatic parenchyma with organized hepatocyte cords surrounding the central lobular vein, interspersed by sinusoid capillaries. However, in the livers of rats in the alcohol group, congestion of the centrilobular vein, hepatocellular ballooning in the areas around the centrilobular veins, microgoticular steatosis, leukocyte infiltrate, and several hepatocytes with pyknotic nuclei were observed (Fig. 1).
Figure 1. Photomicrograph of livers of females in experimental groups. A – B (control); C–D (alcohol); E – F (alcohol + melatonin). Central lobular vein without congestion (long arrow); Central lobular vein with congestion (Vcc); Hepatocyte ballooning (short arrows); leukocyte infiltrate (IL); Multigoticular steatosis (dashed arrows); Hepatocytes with pyknotic nuclei (arrowheads). HE
3.3 LIVER MORPHOMETRIC ANALYSIS
There was an increase in the percentage of lobular parenchyma, promoting a reduction in the non-lobular parenchyma in the liver of rats in the alcohol group. Melatonin prevented this effect, presenting similar parameters in relation to the control group (Table 2).
Table 2. Mean ± standard deviation of the percentage of lobular and non-lobular parenchyma in the liver of females in the experimental groups
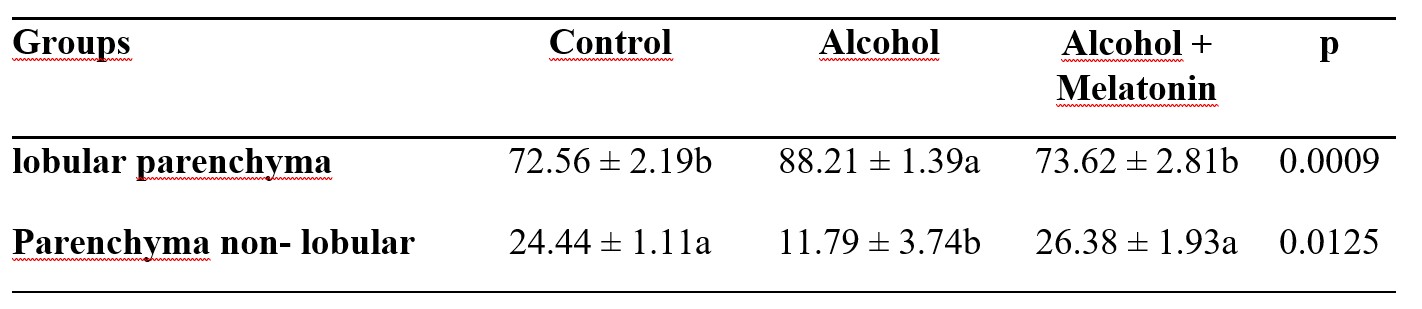
Means followed by the same letter in the lines do not differ significantly by the Tukey and Kramer test (p<0.05).
3.4 LIVER ALTERATION SCORES
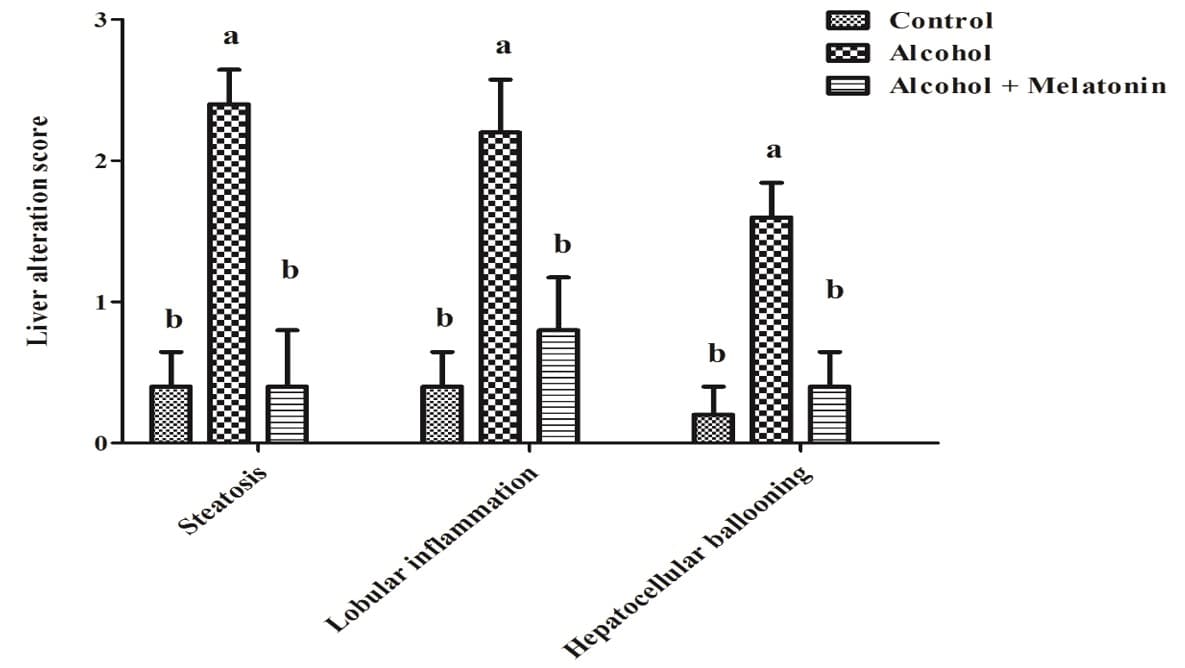
In the semi-quantitative evaluation of the scores of liver alterations, females in the alcohol group revealed high scores of steatosis, lobular inflammation and ballooning hepatocytes. Melatonin treatment considerably reduced these effects, with scores similar to the control group (Fig. 2).
Figure 2. Liver alteration score chart in females of experimental groups. Check for a significant increase in steatosis, lobular inflammation and hepatocellular ballooning. Means followed by the same letter do not differ significantly according to the Tukey and Kramer test (p<0.05)
3.5 LIVER HISTOCHEMISTRY
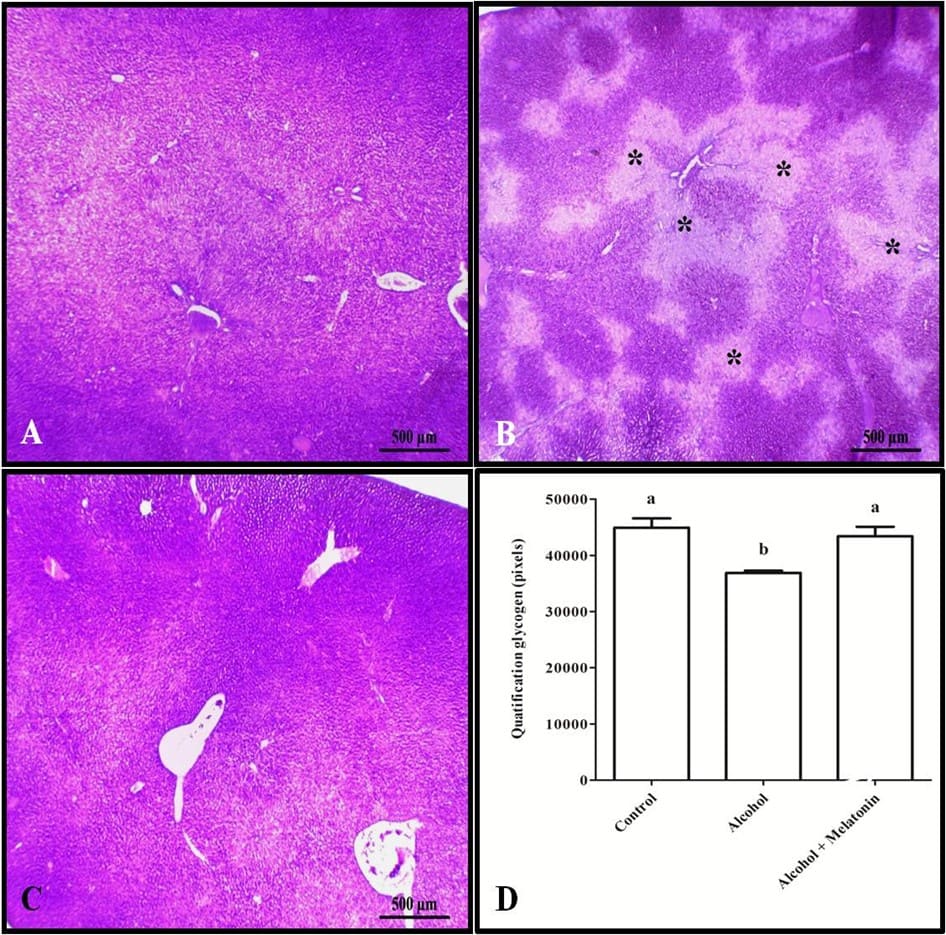
Trichrome staining and quantification in pixels revealed increased collagen in the liver around the veins, characterizing venular fibrosis in females that received only alcohol (Fig. 3). However, with regard to liver glycogen, the animals in this group showed a significant reduction when compared to the other groups (Fig. 4).
Figure 3. Histochemistry for collagen in the liver of females in the experimental groups. A (control); B (alcohol); C (alcohol + melatonin). Note in B greater marking characterizing venular fibrosis (arrow). D – Quantification in pixels. Mallory’s trichrome. Means followed by the same letter do not differ significantly according to the Tukey and Kramer test (p<0.05).
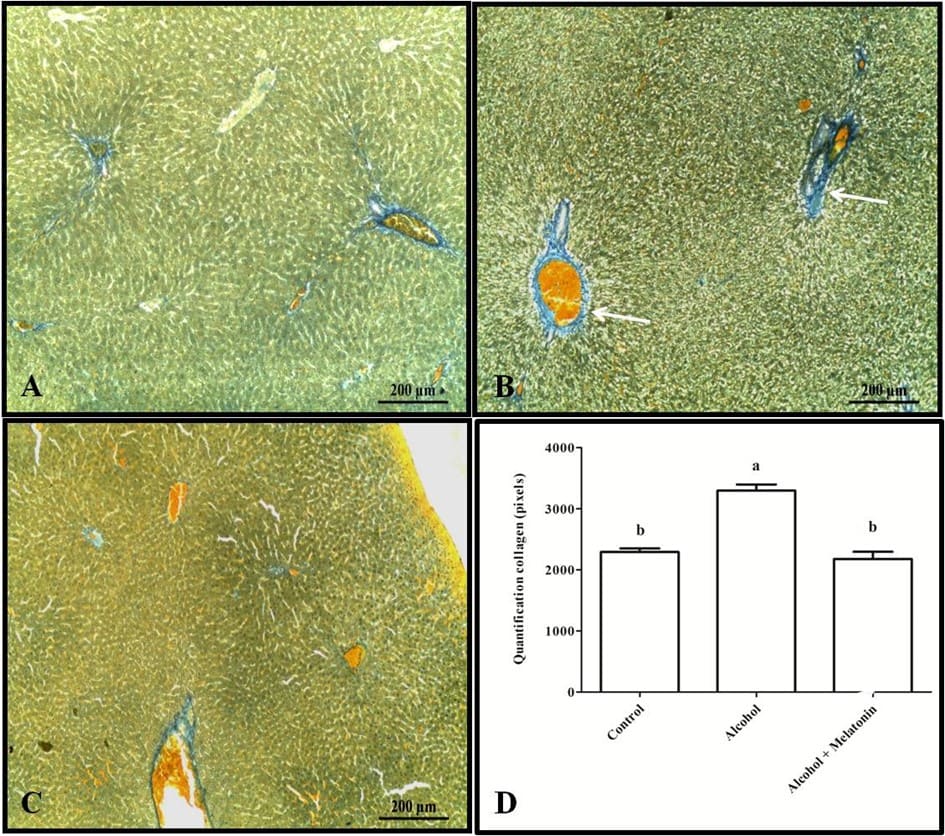
Figure 4. Histochemistry for glycogen in the liver of females in experimental groups. A (control); B (alcohol); C (alcohol + melatonin). Observe in B several less stained areas (*), signaling the absence of glycogen. D – Quantification in pixels. SBP Means followed by the same letter do not differ significantly according to the Tukey and Kramer test (p<0.05)
4. DISCUSSION
The liver is the main organ that suffers the deleterious effects of toxic substances (BHADORIA et al., 2015). Alcohol consumption causes structural changes resulting from oxidative stress. Therefore, it is one of the central mechanisms of liver damage (RADIC et al., 2019). In the present study, it was found that in the alcohol group, the liver showed congestion of the central lobular vein, corroborating the results presented by Radic et al. (2021), where, according to Allaithi; Alazawi (2019) can be explained by the accumulation of fat in the liver, which in turn stimulates an inflammatory response by stellate cells, thus releasing inflammatory cytokines in order to bring a greater amount of white blood cells to the affected region. What would also justify in our findings the presence of leukocyte infiltrate in the hepatic parenchyma. The presence of these infiltrates, mainly neutrophils within the hepatic parenchyma, is a characteristic indicative of alcoholic hepatitis (RAMAIAH; JAESCHKE, 2007) and occurs abundantly in the portal tracts (MOSTAFA et al., 2020).
According to Tao et al. (2021) and Yeh; Brunt (2014), steatosis and hepatocyte ballooning are among alcoholic liver injuries, which was also evidenced in our study. Steatosis is considered the most frequent form of alcoholic liver disease and precedes other alterations, such as steatohepatitis and fibrosis (KISSELEVA; BRENNER, 2019; GAO; BATALLER, 2011). It is characterized by impairment of the hepatic lobular parenchyma resulting from the accumulation of lipids in hepatocytes. This impairment is observed mainly around the central lobular vein, however, with the progression of the pathology, it can involve all areas, and is often accompanied by a chronic inflammatory infiltrate (MOSTAFA et al., 2020), as we could observe in our study. Hepatic ballooning, on the other hand, is the morphological modification due to hepatocellular stress resulting from the oxidation of free fatty acids, and endoplasmic reticulum stress due to fat accumulation, thus promoting injuries to the intracellular environment, such as changes in Perilipin. Damage is seen through morphological changes such as cell swelling, enlargement of hepatocytes, rounding of the cell contour, reticulation and clearing of the cytoplasm from a viscous material called Mallory-Denk bodies or mallory hyaline, which is composed of intermediate filaments well phosphorylated, ubiquitin – binding protein P62 and ubiquitin in the cytoplasm of hepatocytes (MOSTAFA et al., 2020; SCHILD; GUY, 2018).
Steatosis can progress to its inflammatory state, steatohepatitis. In later and more severe cases, the disposition of collagen resulting from parenchymal damage, such as chronic inflammation, necrosis and subsequent cell regeneration, can cause fibrosis in response to exacerbated regenerative activity (MASSEY; ARTEEL, 2012; ROY et al., 2015), which justifies the increase in collagen in the animals in the Alcohol group. It was also verified in our findings, hepatocytes with pyknotic nucleus. According to the literature, this is indicative of low cell activity, suggestive of a cell necrosis process (BHADORIA et al., 2015).
In our research, the ingestion of EtOH also caused a marked decrease in the glycogen reserve of the hepatic parenchymal tissue. In the liver, glucose is transported to hepatocytes by means of transport proteins that are present in its plasma membrane (NANJI; FOGT; GRINIUVIENE 1995), thus promoting the formation of the sugar reserve in the form of glycogen through Glycogen Synthase (GS). The consumption of EtOH does not promote changes in the phosphorylase activity of GS, however, it promotes a reduction in expression levels and in its mRNA (MARTIN et al., 2004; SRINIVASAN; SHAWKY; KAPHALIA, 2019), which causes a decrease in the glycidic reserve total in periportal and perivenous hepatocytes (STEINER; CROWELL; LANG, 2015).
Alcohol+melatonin group showed attenuation of the structural changes found in the liver of animals in the alcohol group, with parameters close to those of the control group. It has already been shown that this indolamine is a splendid non-enzymatic antioxidant (SINGH; JADHAV, 2014), with a high capacity to minimize changes and help maintain the usual arrangement of hepatocytes (GHOSH et al., 2023), in addition to being widely distributed in the body. This is due to its structure having electron donor sites on carbon 2 and 3 of the pyrrole ring, and its amphiphilic nature, that is, lipophilic and hydrophilic, thus providing the ability to easily pass cell membranes and blood-brain barriers (REITER et al., 2013), thereby reducing oxidative stress by scavenging free radicals of all kinds (REITER et al., 2014). Therefore, melatonin may have acted on abnormalities, preventing imbalance in antioxidant and pro-oxidant mechanisms, which in turn may have delayed or prevented the development of lesions resulting from oxidative stress (HU et al., 2009) in the alcohol + group. melatonin.
According to Galano, Tan and Reiter (2011), this indolamine has the ability to promote a reduction in lipid peroxidation and an increase in the concentration of antioxidants by modulating their expression, in addition to the potential to prevent the reduction of glutathione (GSH) when used over a prolonged period, in addition to decreasing and the sensitization of kupffer cells to alcohol, which reduces ROS levels. As a result of this reduction, it indirectly acts on leukocyte infiltrates, promoting an improvement in inflammation (ZHANG et al., 2021). However, melatonin also acts directly on inflammation, as it has the ability to attenuate the migration of neutrophil infiltrates in response to injury (KURHALUK; TKACHENKO 2020). It has already been demonstrated by Ghosh et al. (2023) in an experimental model of oxidative stress in rats, that this indoleamine acts to reduce collagen deposition in the liver, thus preventing fibrosis through and acting to reduce fibroblast proliferation (LI et al., 2020), thus promoting an indirect response in fibrosis by cytokines. As for the reserve of sugar in the liver, the alcohol + melatonin group presented a glycogen reserve close to the control group. Previous studies have pointed out that melatonin has the ability to increase hepatic glycogen content (FARIA et al., 2022), and this increase can be explained by the improvement in tissue glucose uptake and/or insulin sensitivity through a dependent pathway. of Akt activated via melatonin receptor (SHIEH et al., 2009). Thus, it can prevent the reduction of glycogen reserves observed in the group subjected to chronic alcohol consumption.
5. CONCLUSIONS
The results of the present study demonstrated that melatonin has great therapeutic potential in preventing damage to the liver of adolescent rats subjected to immoderate alcohol consumption, in addition to positive effects on the deposition of collagen and glycogen in the liver. However, it is necessary to deepen studies in this area to obtain more data that prove and reinforce the potential of this hormone in the preventive therapy of alterations and pathologies caused by chronic alcohol consumption.
BIBLIOGRAPHIC REFERENCES
ABD-ALLAH, A. R. A. et al. Effect of melatonin on estrogen and progesterone receptors in relation to uterine contractions in rats. Pharmacological Research , v . 47, pp. 349–354, 2003. DOI: 10.1016/s1043-6618(03)00014-8.
ALLAITHI, L. A. A.; Al-AZAWI, W. M. K. Study of the effect of salvia officinalis leaves extract and xenical drug on some of the biochemical and histological parameters in the rats induced with hyperlipidemia. Plant Archives, v . 19, pp. 1111–1122, 2019.
AMARAL, F. G. et al. New insights into the function of melatonin and its role in metabolic disturbances. Expert Review of Endocrinology & Metabolism, v. 14, no. 4, pp. 293–300, 2019. DOI: 10.1080/17446651.2019.1631158.
ANDRADE, A. G.; SIU, E. R. Alcohol and the health of Brazilians: panorama 2019. Health and alcohol information center. P. 104, 2019.
ARAÚJO-FILHO, J. L. S. et al. Histomorphometric analysis of the heart of rats indirectly exposed to ethanol and chronic malnutrition during the perinatal period. Journal of Medical and Biological Sciences, v. 6, no. 1, pp. 17-25, 2007. DOI: 10.9771/cmbio.v6i1.4144.
BEST, C. A.; LAPOSATA, M. Fatty acid ethyl esters toxic non-oxidative metabolites of ethanol and markers of ethanol intake. Frontiers in Bioscience, v. 8, no. 5, pp. e202-217, 2003. DOI: 10.2741/931.
BHADORIA, P. et al. Effect of Ethephon on the Liver in Albino Rats: A Histomorphometric Study. Biomedical Journal, v. 38, no. 5, pp. 421–427, 2015. DOI: 10.4103/2319-4170.155589.
BROCARDO, P. S.; GIL-MOHAPEL, J.; CHRISTIE, B. R. The role of oxidative stress in fetal alcohol spectrum disorders. brain Research Reviews, vol. 67, no. 1–2, pp. 209–225, 2011. DOI: 10.1016/j.brainresrev.2011.02.001.
ENGELMAN, M. F. B. et al. Morphometric study of the liver of rats submitted to supraphysiological doses of thyroxine. Brazilian Archives of Endocrinology & Metabolism, v. 45, no. 2, pp. 173-179, 2001. DOI: 10.1590/S0004-27302001000200009.
FARIA, V. S. et al. Melatonin Potentiates Exercise-Induced Increases in Skeletal Muscle PGC-1 α and Optimizes Glycogen Replenishment. Frontiers in Physiology, vol. 13, pp. 1–11, 2022. DOI: 10.3389/fphys.2022.803126.
FARKAS, Á.; KEMÉNY, L. Alcohol, Liver, Systemic Inflammation and Skin: A Focus on Patients with Psoriasis. Skin Pharmacology and Physiology, v. 26, no. 3, pp. 119–126, 2013. DOI: 10.1159/000348865.
FIOCRUZ. III national survey on drug use by the Brazilian population. [sl .: sn], 2017. Available at: <https: //www.arc a .fiocruz.br/bitstream/icict/34614/1/III%20LNUD_PORTUGU%C3%8 AS.pdf>.
GALANO, A.; TAN, D. X; REITER, R. J. Melatonin as a natural ally against oxidative stress: a physicochemical examination. Journal of Pineal Research, vol. 51, no. 1, pp. 1-16, 2011. DOI: 10.1111/j.1600-079X.2011.00916.x.
GALANO, A.; TAN, D. X.; REITER, R. J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules, v. 23, no. 3, pp. 530, 2018. DOI: 10.3390/molecules23030530.
GAO, B.; BATALLER, R. Alcoholic Liver Disease: Pathogenesis and New Therapeutic Targets. GASTROENTEROLOGY, v. 141, no. 5, pp. 1572-1585, 2011. DOI: 10.1053/j.gastro.2011.09.002.
GHOSH, P. et al. Insights into the antioxidative mechanisms of melatonin in ameliorating chromium-induced oxidative stress-mediated hepatic and renal tissue injuries in male Wistar rats. Food and Chemical Toxicology, v. 173, pp. 113630, 2023. DOI: 10.1016/j.fct.2023.113630.
GUNATA, M.; PARLAKPINAR, H.; ACET, H. A. Melatonin: A review of its potential functions and effects on neurological diseases. Revue Neurologique, vol. 176, no. 3, pp. 148–165, 2020. DOI: 10.1016/j.neurol.2019.07.025.
HARDELAND, R.; PANDI-PERUMAL, S. R.; CARDINALI, D. P. Melatonin. The International Journal of Biochemistry & Cell Biology, v. 38, no. 3, pp. 313-316, 2006. DOI: 10.1016/j.biocel.2005.08.020.
HU, C. et al. Protective role of melatonin in early-stage and end-stage liver cirrhosis. Journal of Cellular and Molecular Medicine, v. 23, no. 11, pp. 7151–7162, 2019. DOI: 10.1111/jcmm.14634.
HU, S. et al. Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. European Journal of Pharmacology, v. 616, pp. 287–292, 2009. DOI: 10.1016/j.ejphar.2009.06.044.
KISSELEVA, T.; BRENNER, D. A. The Crosstalk between Hepatocytes, Hepatic Macrophages, and Hepatic Stellate Cells Facilitates Alcoholic Liver Disease. Cell Metabolism, v. 30, no. 5, pp. 850-852, 2019. DOI: 10.1016/j.cmet.2019.10.010.
KLEINER, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology, v. 41, no. 6, pp. 1313–1321, 2005. DOI: 10.1002/hep.20701.
KURCER, Z. et al. Melatonin improves methanol intoxication-induced oxidative liver injury in rats. Journal of Pineal Research, vol. 43, no. 1, pp. 42-49, 2007. DOI: 10.1111/j.1600-079X.2007.00441.x.
KURHALUK, N.; TKACHENKO, H. Melatonin and alcohol-related disorders. Chronobiology International, v. 37, no. 6, pp. 781–803, 2020. DOI:10.1080/07420528.2020.1761372.
KURHALUK, N.; TKACHENKO, H.; LUKASH, O. Melatonin modulates oxidative phosphorylation, hepatic and kidney autophagy-caused subclinical endotoxemia and acute ethanol-induced oxidative stress. Chronobiology International, v. 37, no. 12, pp. 1709–1724, 2020. DOI: 10.1080/07420528.2020.1830792.
LI, N. et al. Melatonin ameliorates renal fibroblast-myofibroblast transdifferentiation and renal fibrosis through miR-21-5p regulation. Journal of Cellular and Molecular Medicine, v. 24, no. 10, pp. 5615–5628, 2020. DOI: 10.1111/jcmm.15221.
MAHIEU, S. et al. Melatonin reduces oxidative damage induced by aluminum in rat kidney. Toxicology Letters, v. 190, no. 1, pp. 9-15, 2009. DOI: 10.1016/j.toxlet.2009.06.852.
MARCO, I. N. et al. Long-Term Effects of Intermittent Adolescent Alcohol Exposure in Male and Female Rats. Frontiers in Behavioral Neuroscience, v. 11, no. 233, pp. 1-13, 2017. DOI: 10.3389/fnbeh.2017.00233.
MARTIN, J. V. et al. Effects of dietary caffeine and alcohol on liver carbohydrate and fat metabolism in rats. Medical science monitor: international medical journal of experimental and clinical research, v. 10, no. 12, pp. BR455-BR461, 2004.
MASSEY, V. L.; ARTEEL, G. E. Acute Alcohol-Induced Liver Injury. Frontiers in Physiology, vol. 3 n. 193, pp. 1-8, 2012. DOI: 10.3389/fphys.2012.00193.
MINCIS, M. et al. Alcohol and the Liver. GED: Gastroenterology Endoscopy digestive, v. 30, no. 4, pp. 152-162, 2011.
MOSTAFA, M. et al. Fatty Liver Disease: A Practical Approach. Archives of Pathology & Laboratory Medicine, v. 144, no. 1, pp. 62–70, 2020. DOI: 10.5858/arpa.2019-0341-RA.
MOUSTAFA, A. M. et al. Effect of bromocriptine on uterine contractility in near term pregnant rats. Pharmacological Research, v. 39, no. 2, p. 89-95, 1999. DOI: 10.1006/phrs.1998.0399.
NANJI, A. A.; FOGT, F.; GRINIUVIENE, B. Alterations in Glucose Transporter Proteins in Alcoholic Liver Disease in the Rat. The American Journal of Pathology, v. 146, no. 2, pp. 329-334, 1995.
NORBERG, A. et al. Role of Variability in Explaining Ethanol Pharmacokinetics: Research and Forensic Applications. Clinical Pharmacokinetics, v. 42, no. 1, p. 1–31, 2003. DOI: 10.2165/00003088-200342010-00001.
NWOZO, S.; AJAGBE, A.; OYINLOYE, B. Hepatoprotective effect of Piper guineense aqueous extract against ethanol-induced toxicity in male rats. Journal of Experimental and Integrative Medicine, v. 2, no. 1, pp. 71-76, 2012. DOI: 10.5455/jeim.241111.or.016.
PAGET, G. E.; BARNE, J. M. Evaluation of results: quantitative application in different species. Pharmacometrics , vol. 1. p. 161. 9th ed. New York: Academic Press; 1994.
POEGGELER, B. et al. Melatonin, hydroxyl radical- mediated oxidative damage, and aging: A hypothesis. Journal of Pineal Research, v. 14, no. 4, pp. 151–168, 1993. DOI: 10.1111/j.1600-079x.1993.tb00498.x.
RADIC, I. et al. Protective effects of whey on rat liver damage induced by chronic alcohol intake. Human & Experimental Toxicology, v . 38, no. 6, pp. 632–645, 2019. DOI: 10.1177/0960327119829518.
RADIC, I. et al. Protective effects of pumpkin (Cucurbita pepo L.) seed oil on rat liver damage induced by chronic alcohol consumption. Archives of Biological Sciences, vol. 73, no. 1, pp. 123–133, 2021. DOI: 10.2298/ABS201205008R.
RAMAIAH, S. K.; JAESCHKE, H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicology Mechanisms and Methods, v.17, n. 7, pp. 431– 440, 2007. DOI: 10.1080/00952990701407702.
REITER, R. J. et al. Peripheral Reproductive Organ Health and Melatonin: Ready for Prime Time”, International Journal of Molecular Sciences, v. 14, no. 4, pp. 7231-7272, 2013. DOI: 10.3390/ijms14047231.
REITER, R. J.; TAN, D. X.; GALANO, A. Melatonin: exceeding expectations. Physiology (Bethesda, Md.), v. 29, no. 5, pp. 325–333, 2014. DOI: 10.1152/physiol.00011.2014.
ROCCO, A. et al. Alcoholic disease: Liver and beyond. World Journal of Gastroenterology, v. 20, no. 40, pp. 14652-14659, 2014. DOI: 10.3748/wjg.v20.i40.14652.
ROY S. et al. The role of miRNAs in the regulation of inflammatory processes during hepatofibrogenesis. Hepatobiliary Surgery and Nutrition, v. 4, no. 1, p. 24-33, 2015. DOI: 10.3978/j.issn.2304-3881.2015.01.05.
SCHEIDT, L. et al. Ethanol during adolescence decreased the BDNF levels in the hippocampus in adult male Wistar rats, but did not alter aggressive and anxiety -like behaviors. Trends Psychiatry Psychother. v. 7, no. 3, p. 143-151, 2015. DOI: 10.1590/2237-6089-2015-0017.
SCHILD, M. H.; GUY, C. D. Nonalcoholic Steatohepatitis. Surgical Pathology Clinics, v. 11, no. 2, pp. 267–285, 2018. DOI: 10.1016/j.path.2018.02.013.
SHIEH, J. M. et al. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKC ζ -Akt-GSK3 β pathway in hepatic cells. Journal of Pineal Research, v. 47, no. 4, pp. 339–344, 2009. DOI: 10.1111/j.1600-079X.2009.00720.x.
SINGH, M., JADHAV, H. R. Melatonin: functions and ligands. Drug Discovery Today, v. 19, no. 9, pp. 1410-1418, 2014. DOI: 10.1016/j.drudis.2014.04.014.
SRINIVASAN, M. P.; SHAWKY, N. M.; KAPHALIA, B. S. Alcohol-induced ketonemia is associated with lowering of blood glucose, downregulation of gluconeogenic genes, and depletion of hepatic glycogen in type 2 diabetic db/db mice. Biochemical Pharmacology, v. 160, pp. 46-61, 2019. DOI: 10.1016/j.bcp.2018.12.005.
STEINER, J. L.; CROWELL, K. T.; LANG, C. H. Impact of Alcohol on Glycemic Control and Insulin Action. Biomoleculas, v. 5, no. 4, pp. 2223-2246, 2015. DOI: 10.3390/biom5042223.
TAO, Z. et al. Echinacoside ameliorates alcohol-induced oxidative stress and hepatic steatosis by affecting SREBP1c/FASN pathway via PPAR α. Food and Chemical Toxicology, v.148, pp. 1-9, 2021. DOI: 10.1016/j.fct.2020.111956.
ULLAH, U. et al. Hepatoprotective effects of melatonin and celecoxib against ethanol-induced hepatotoxicity in rats. Immunopharmacology and Immunotoxicology, v. 42, no. 3, pp. 255–263, 2020. DOI: 10.1080/08923973.2020.1746802.
VARLINSKAYA, E. I.; SPEAR, L. P.; SPEAR, N. E. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clinical & Experimental Research, v. 25, no. 3, pp. 377–385, 2001. DOI: 10.1111/j.1530- 0277.2001.tb 02224.x.
VONGHIA, L. et al. Acute alcohol intoxication. European Journal of Internal Medicine, v. 19, no. 8, pp. 561–567, 2008. DOI: 10.1016/j.ejim.2007.06.033.
WHO – World Health Organization. Global status report on alcohol and health 2018. 2018, p. 472.
YANG, L. et al. Chronic Alcohol Exposure Increases Circulating Bioactive Oxidized Phospholipids. Journal of Biological Chemistry, v. 285, no. 29, pp. 22211–22220, 2010. DOI: 10.1074/jbc.M110.119982.
YEH, M. M.; BRUNT, E. M. Pathological features of fatty liver disease. Gastroenterology, v. 147, no. 4, pp. 754–764, 2014. DOI: 10.1053/j.gastro.2014.07.056.
ZHANG, H. et al. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. Journal of Hazardous Materials, v. 407, pp. 1-49, 2021. DOI: 10.1016/j.jhazmat.2020.124489.
[1] PhD student in Animal Bioscience – UFRPE. ORCID: 0000-0001-9404-7501. Curriculum Lattes: http://lattes.cnpq.br/8213260513385508.
[2] Bachelor’s Degree in Biological Sciences – UFRPE. ORCID: 0000-0003-3659-3947. Lattes curriculum: https://lattes.cnpq.br/5069796237775832.
[3] Master in Agricultural Entomology – UFRPE. ORCID: 0000-0003-3822-3050. Curriculum Lattes: http://lattes.cnpq.br/4008994020879541.
[4] Doctoral student in the Postgraduate Program in Animal Bioscience – UFRPE. ORCID: 0000-0002-4733-461X. Curriculum Lattes: http://lattes.cnpq.br/1906334502843226.
[5] Doctoral student in the Postgraduate Program in Animal Bioscience – UFRPE. ORCID: 0000-0002-6228-6951. Curriculum Lattes: http://lattes.cnpq.br/1783975917572458.
[6] PhD in Animal Bioscience – UFRPE. ORCID: 0000-0002-2507-3682 Lattes CV: http://lattes.cnpq.br/9465720906397764.
[7] Master in Gerontology – UFSM. ORCID: 0000-0001-9528-7312. Curriculum Lattes: http://lattes.cnpq.br/9959642679541707.
[8] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: 0000-0003-1493-7964. Curriculum Lattes: http://lattes.cnpq.br/9044747136928972.
[9] PhD in morphology – UNIFESP. ORCID: 0000-0001-5940-9220. Curriculum Lattes: http://lattes.cnpq.br/1539131079574469.
[10] Advisor. PhD in Nuclear Technology – USP. ORCID: 0000-0001-9533-5476 Lattes CV: http://lattes.cnpq.br/4292195468804301.
Submitted: July 11, 2023.
Approved: August 14, 2023.