ORIGINAL ARTICLE
MELO, Fernando Henrique Boaventura de [1], TEIXEIRA, Valéria Wanderley [2], CAMARA, Claudio Augusto Gomes da [3], SOUZA, Catiane Oliveira [4], CRUZ, Glaucilane dos Santos [5], MONTEIRO, Vaneska Barbosa [6], MORAES, Marcilio Martins [7], VIEIRA FILHO, Leucio Duarte [8], MALERBO-SOUZA, Darclet Teresinha [9], SOARES, Anísio Francisco [10], TEIXEIRA, Álvaro Aguiar Coelho [11]
MELO, Fernando Henrique Boaventura de et al. Comparative immunotoxicity of bees, apis mellifera (hymenoptera: apidae), exposed to natural and synthetic xenobiotics. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 09, Ed. 02, Vol. 02, pp. 43-65. February 2024. ISSN: 2448-0959, Acess link: https://www.nucleodoconhecimento.com.br/biology/immunotoxicity-of-bees, DOI: 10.32749/nucleodoconhecimento.com.br/biology/immunotoxicity-of-bees
ABSTRACT
The objective of this study was to compare the effects of natural and synthetic chemical pesticides, thereby examining the supposed selectivity of these natural compounds on Apis mellifera bees. The LC50 values used in the bioassays were obtained from the research by Souza et al. (2023) and are as follows: Karate® (13.4 µL/100 mL), Limonene compound (1,440 µL/100 mL), and Roundup® (712,290 µL/100 mL). However, as reported by these authors, a concentration of 250 µL/100 ml was used for Azamax®. These LC50 values, along with a concentration of 250 µL/100 mL of Azamax®, were employed in immunohistochemical analyzes using the TUNEL method and PCNA in the midgut of bees. Immunological assessments (nitric oxide, phenoloxidase, and oxidative stress markers TBARS and GSH) were also conducted on adult worker bees. The xenobiotic treatments did not reveal apoptosis or cell proliferation. Nonetheless, we observed epithelial degeneration, marked by the presence of vacuolated cells, suggesting a necrotic process. Except for the Limonene compound, all substances induced oxidative stress, leading to increased levels of TBARS. Although there were no differences in GSH levels, we observed alterations in the immune system of these insects, characterized by increased phenoloxidase activity and NO2 levels. Based on the acquired results, it is possible to conclude that caution should be exercised when using chemical pesticides in agriculture, whether they are of synthetic or natural origin, as they have the potential to cause irreversible histopathological and immunological damage. This study also underscores the importance of conducting more comprehensive investigations into the impact of natural products on the physiology of pollinator insects.
Key words: Apis mellifera, Xenobiotics, Immunohistochemistry, Oxidative stress, Phenoloxidase.
1. INTRODUCTION
Honey bees, Apis mellifera Linnaeus (Hymenoptera: Apidae), play a prominent role in ecosystems due to their involvement in pollination, a role they perform both spontaneously in natural environments and within anthropomorphized settings. They facilitate the transfer of reproductive cell particles, thus contributing to the genetic diversity of both wild and cultivated plant species (Klein, et al. 2007; Michener, 2007; Potts, et al. 2016; Eardley, et al. 2016). As arthropods of great significance, honey bees intrinsically contribute to the survival of numerous organisms on Earth (Potts et al., 2016; Eardley et al., 2016). Despite their environmental and economic importance (Giannini et al., 2015), recent years have seen a decline in bee populations and a reduction in species diversity on all continents (Zattara & Aizen, 2021).
Agricultural pesticides impact honey bee populations in several ways and are identified as one of the multifactorial causes contributing to the phenomenon known as Colony Collapse Disorder (CCD), which affects social bees of the genus Apis and native species (Graystock et al., 2013; Goulson et al., 2015). Neurotoxic insecticides, such as neonicotinoids and pyrethroids, for example, are not only lethal but also capable of inducing behavioral changes in worker bees (Decourtye et al., 2005; Gill & Raine, 2014). Additionally, the widely used herbicide glyphosate has been found to reduce the learning abilities of bees (Herbert et al., 2014).
From an immunological perspective, Gregorc & Ellis (2011) conducted tests on A. mellifera larvae using chemical pesticides—organophosphate, neonicotinoid, and the herbicide glyphosate. They observed an increase in apoptosis in the midgut, with respective percentages of 61%, 65%, and 69% of cell death in the treated larvae compared to 10% in the control group. This demonstrates that the pesticides exert an effect at the cellular level. Apoptosis is the process of programmed cell death that occurs naturally in organisms to maintain cell homeostasis and serve as a defense mechanism (Ashe & Berry, 2003). However, depending on an individual’s capacity, it can recover through the process of cell proliferation (Caccia et al., 2019).
Oxidative stress has the potential to initiate the process of cell death and occurs due to the balance between reactive oxygen or nitrogen species (ROS and RNS) and antioxidant compounds (Maulik et al., 1998; Barbosa et al., 2010). ROS and RNS are free radicals that, when in balance, play important roles in the immune system of insects. An example of this is nitric oxide, which participates in processes signaling the presence of entomopathogens and xenobiotics, and is part of the humoral defense mechanisms of these arthropods (Bartling et al., 2021). Phenoloxidase is another component of this defense, as this enzyme participates in the formation of melanin, which is involved in the sclerotization of the cuticle, wound healing, and the recognition of foreign agents (Negreiro et al., 2004).
In this context, over the last two decades, research on botanical biopesticides has significantly increased due to several advantages they offer, including greater selectivity towards non-target organisms (Isman, 2006; Isman, 2020). However, despite the extensive body of research, little attention has been given to the effects of these natural insecticides on insect physiology (Isman, 2020; Giannini et al., 2015). This is a cause for concern, as studies have revealed that essential oils and their isolated compounds have the potential to impact the life history of insects, affecting pests as well as natural enemies and leading to histopathological (Almehmadi, 2011; Oliveira et al., 2021), nutritional (Cruz et al., 2017; Oliveira et al., 2021), and immunological changes (Silva et al., 2020).
Thus, physiological tests are necessary to ensure the safety of these substances on bees in agricultural fields. Bee workers can potentially contaminate themselves, and consequently the entire colony, either during foraging or through residues in the water in the case of synthetic chemical pesticides (Mullin et al., 2010; Potts et al., 2010; Ruiz-Toledo & Sánchez-Guillén, 2014).
Given the context provided, this research aimed to compare the immune responses of A. mellifera bees when exposed to synthetic xenobiotics (pyrethroid insecticide – Karate® and glyphosate herbicide – Roundup®) and natural xenobiotics (biopesticide – Azamax® and Limonene compound) commonly used in agriculture.
2. MATERIAL AND METHODS
2.1 OBTAINING INSECTS
The honey bees used in this research were obtained from the experimental apiary of the Department of Animal Science at the Federal Rural University of Pernambuco (UFRPE). Workers bees were collected using plastic containers with perforated lids placed at the hive entrances. Subsequently, the insects were transported to the Laboratory of Insect Physiology (LAFI) and the Laboratory of Morphological Studies in Vertebrates and Invertebrates (LABEMOVI), both at UFRPE, where they were utilized in the bioassays.
2.2 CONCENTRATIONS USED IN BIOASSAYS
The Lethal Concentrations 50 (LC50) used for immunohistochemical and immunological evaluations of the A. mellifera bee population from the UFRPE apiary were obtained from Souza et al. (2023). They reported LC50 values as follows: 13.4 µL/100 mL for the Karate® insecticide (pesticide + honey solution 1:1 + 1% Dimethylsulfoxide (DMSO)), 1,440 µL/100 mL for the Limonene compound (honey solution + DMSO), and 712,290 µL/100 mL for the Roundup® herbicide (honey + water + DMSO). Regarding the botanical insecticide Azamax®, a concentration of 250 µL/100 mL of the solution (honey + DMSO) was used. As demonstrated by Souza et al. (2023), this concentration represented the highest level of the product that resulted in mortality without repelling insects; higher concentrations repelled 100% of the sampled individuals, making it impossible to determine the LC50 for Azamax®. The control group was treated solely with the honey solution (1:1) + 1% DMSO. After 24 hours of exposure to the treatments, the surviving bees were used in the subsequent tests.
2.3 APIS MELLIFERA MIDGUT APOPTOSIS
For immunohistochemical analysis, including apoptosis and cell proliferation, midguts were embedded in paraffin, and 5 µm-thick sections were obtained using a Minot microtome (Leica, 2035). These sections were then placed in a water bath and collected on silanized slides. Images were captured and digitized using Leica LAS Image software (Junqueira & Junqueira, 1983; Solomon, 2009). To detect apoptosis via DNA fragmentation, silanized slides containing midgut sections underwent the TUNEL test (Terminal Deoxynucleotidyl Transferase Uracil Nick End Labeling) Costa (2022); Gavrieli (1992), following the Apoptag Plus kit protocol (Merck®). The cuts were initially deparaffinized and hydrated, followed by a 5-minute incubation in PBS (Phosphate Buffered Saline) at room temperature. Proteinase K was then applied to the slides for 15 minutes. After washing in distilled water, the slides were incubated in hydrogen peroxide for 5 minutes at room temperature. Sections were washed in PBS and incubated in an equilibration buffer for 60 minutes at 4°C. Subsequently, sections were incubated in TdT at 37°C for 1 hour in a humid chamber. The stop solution was applied for 10 minutes at room temperature, followed by washing in PBS and incubation in anti-digoxigenin. The slides were rinsed in PBS, and the sections were developed with diaminobenzidine chromogenic substrate (DAB, DakoCytomationTM) for approximately 20 minutes. They were then counterstained with hematoxylin for 20 to 30 seconds. Following this, the slides were washed in running water, dehydrated in increasing alcohol concentrations, and placed in xylene for mounting and observation under a light microscope.
2.4 CELL PROLIFERATION OF THE MIDGUT
To assess cell proliferation, the paraffin was removed from sections, which were then hydrated and subjected to antigen retrieval using citrate buffer (pH=6) in a water bath for 20 minutes at 100°C. After allowing them to rest for 20 minutes at room temperature, a 3% hydrogen peroxide solution was applied for 30 minutes. Next, the sections were rinsed in Tris buffer (Tris-(hydroxymethyl)-aminomethane) and exposed to PCNA antibody (nuclear antigen of cell proliferation) (Spring) at a 1:100 dilution for 1 hour at room temperature. Sections were washed again in Tris buffer and incubated with histofine for 30 minutes. They were subsequently subjected to diaminobenzidine chromogen (DAB, DakoCytomation™) and counterstained with hematoxylin. The apoptotic index and cell proliferation were determined based on the percentage of positive cells, calculated from the count of at least 500 nuclei per treatment, subdivided into 10 randomly selected fields using the 40x objective (Losa et al., 2000; Wu et al., 2013).
2.5 PREPARATION OF THE MACERATE
An adult bee was macerated in 500µL of phosphate buffer (0.1 mM, pH 7.4) within a crucible using a porcelain pestle. The resulting macerate was then transferred to a 2 mL centrifuge tube and centrifuged for 1 minute at 1000 rpm to remove any fragments of the insects’ exoskeleton. The supernatant was subsequently transferred to another 2 mL tube and kept refrigerated at -20°C until analysis. Each individual bee was treated as one repetition, and each treatment consisted of 10 repetitions conducted within 24 hours after application.
2.6 NITRIC OXIDE MEASUREMENT
The Griess reagent Green et al. (1981) was employed to assess the concentration of the nitrite ion (NO2). In a 50 μL aliquot of the macerate, 70 μL of 1% sulfanilamide in 5% phosphoric acid (H3PO4) was added. After incubation, an additional 50 μL of 0.1% naphthylethyleneamine dihydrochloride (NEED) was added to the 50 μL of the sample (macerate/sulfanilamide) in a microtiter plate (Faraldo et al. 2005). The absorbance reading was conducted at 562 nm using the Biochrom Anthos 2010 microplate reader (São Paulo, Brazil), with the ADAP program operating in endpoint mode. Data underwent ANOVA, and means were compared using the Tukey HSD test, with analysis conducted using SAS Institute software.
2.7 PHENOLOXIDASE ACTIVITY
The analysis for each treatment was conducted using 10 μL of the macerate, with each treatment consisting of ten replicates. Duplicates of 50 µL from this mixture were transferred to the microtiter plate. Subsequently, 50 microliters of L-DOPA (L-dihydroxyphenylalanine) at a concentration of 4 g/L (Sigma-Aldrich, St. Louis, MO, USA) were added to each well to activate the enzyme. The absorbance readings were performed using a Biochrom Anthos 2010 microplate reader (São Paulo, Brazil) at 492 nm, with the ADAP program set to kinetic photometry mode. Enzyme activity was measured during the linear phase of the reaction at intervals of 60 seconds for 20 minutes, following the method described by (Faraldo et al., 2006). The data were subjected to the Kruskal-Wallis non-parametric test at a significance level of 5%, using SAS Institute software.
2.8 OXIDATIVE STRESS
To assess oxidative stress, lipid peroxidation was evaluated by measuring thiobarbituric acid (TBARS) reactive substances following the method of (Ohkawa et al. 1979). Ten adult insects from each treatment were homogenized in an ice bath using 1.15% KCl + 3 mM EDTA. Subsequently, the reaction medium was prepared by adding 0.3% thiobarbituric acid, 0.4% Sodium Dodecyl Sulfate (SDS), and 7.5% acetic acid (pH 3.5). The mixture was then heated at 95°C for one hour. After centrifugation, the supernatant’s absorbance was measured at a wavelength of 535 nm. The data obtained were normalized by the protein concentration of the homogenate, as measured according to (Lowry et al., 1951). These data were analyzed using the Kruskal-Wallis non-parametric test at a significance level of 5%, using SAS Institute software. Reduced Glutathione (GSH) levels were determined by measuring non-protein sulfhydryl groups, following the methodology of (Sedlak & Lindsay, 1968). From the homogenate obtained for lipid peroxidation assessment, 80 to 160 mg of tissue were precipitated in a 5% TCA solution. Then, a volume of the supernatant was mixed with a reaction medium containing TRIS 4mM, EDTA 4mM, and DTNB 4mM at pH 8.9. The reaction was incubated at room temperature for 5 minutes, and the absorbance was measured at 412 nm. The result was adjusted for the protein concentration of the homogenate. Data regarding GSH levels assumed normality and homogeneity and were analyzed using ANOVA, with means compared using Tukey’s test at a significance level of 5%, as determined by the SAS Institute.
3. RESULTS
3.1 APIS MELLIFERA MIDGUT APOPTOSIS
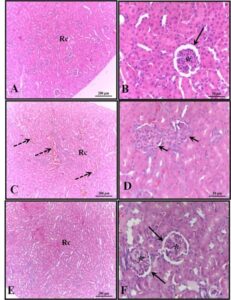
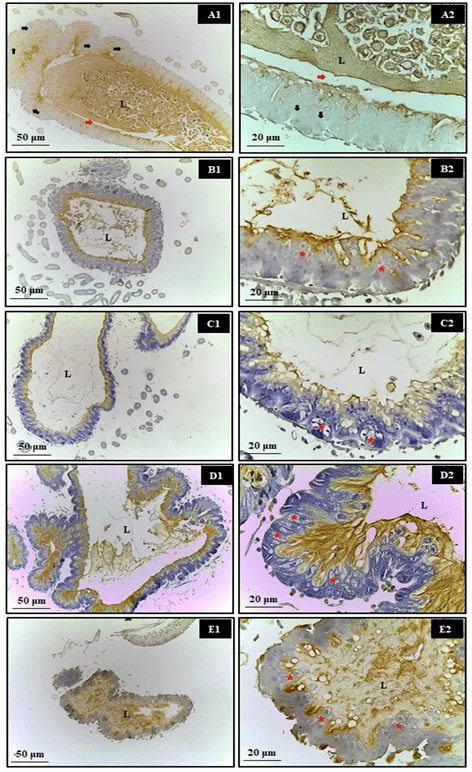
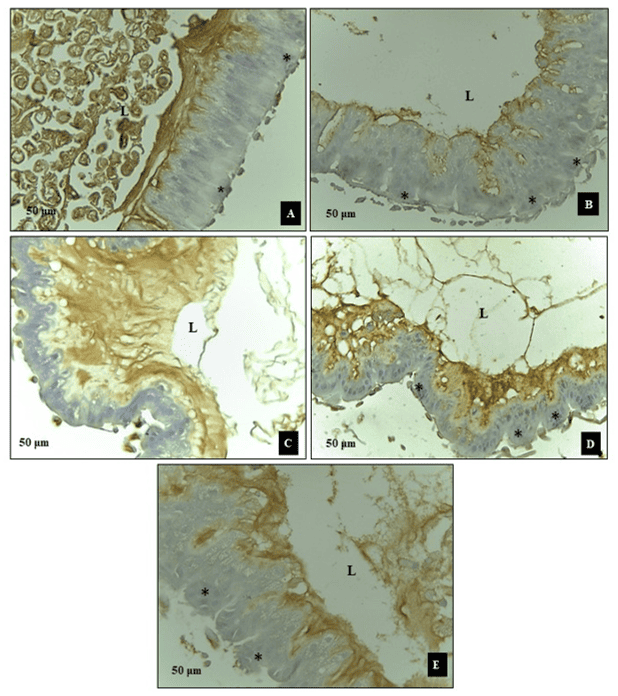
Regarding the immunohistochemical evaluation of the bees’ midgut, no apoptotic nuclei were observed following exposure to synthetic (Roundup® and Karate®) and natural (Azamax® and Limonene compound) xenobiotics after 24 hours. However, the epithelial tissue exhibited cellular degeneration characterized by the presence of cytoplasmic vacuoles, suggesting a necrotic process. It is believed that this cell damage may have been a significant factor preventing these cells from secreting the peritrophic matrix, leading them to come into contact with the contents of the lumen and, consequently, the digestive enzymes (Figure 1).
Figure 1. Evaluation of apoptosis in the midgut of Apis mellifera adults, using the Tunel test. In which, A1 and A2- Control, B1 and B2- Azamax®, C1 and C2- Limonene, D1 and D2- Roundup®, E1 and E2Karate®, L- Lumen, Black arrow- apoptotic nuclei, Red arrow- matrix peritrophic, Asterisk- Vacuolated cells
3.2 CELL PROLIFERATION OF THE MIDGUT
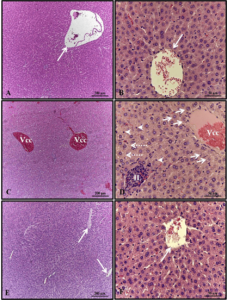
Epithelial cells did not demonstrate activation of regeneration mechanisms by the cell proliferation process in bees exposed to xenobiotics (Figure 2), with positive PCNA being identified only in the control group (Figure 2A). Epithelial cells did not exhibit signs of activation of regeneration mechanisms through the cell proliferation process in bees exposed to xenobiotics (Figure 2). Positive PCNA staining was only observed in the control group (Figure 2A).
Figure 2. Analysis of cell proliferation (PCNA). Cross section of the midgut of adult bees, Apis melifera. A- Control, B- Azamax®, C- Limonene, D- Roundup®, E- Karate®, L- Lumen, asterisk- regenerative cells
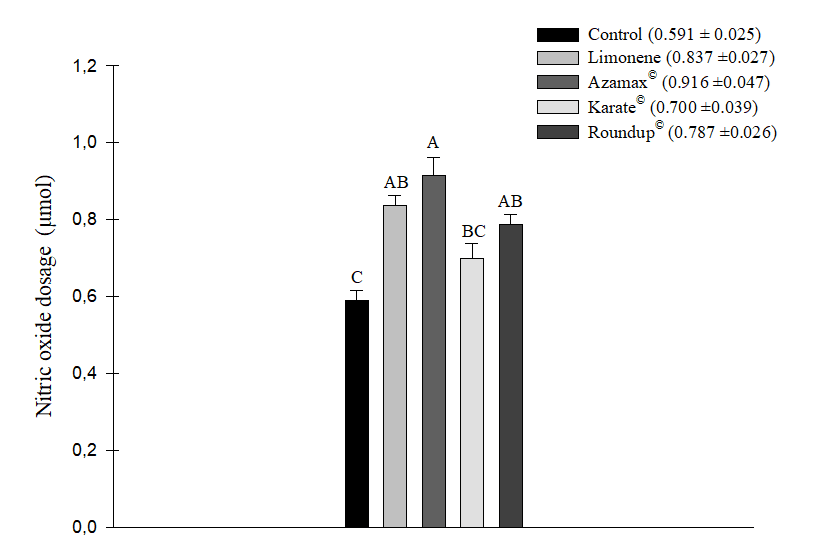
3.3 NITRIC OXIDE MEASUREMENT
After statistical analysis, it was observed that all the chemical products used, namely Karate® (0.700 ± 0.039 µM of NO2), Limonene (0.837 ± 0.027 µM of NO2), Azamax® (0.916 ± 0.047 µM of NO2), and Roundup® (0.787 ± 0.026 µM of NO2), increased the nitric oxide levels in bees. However, the most significant increase was observed in the Azamax® treatment. Limonene did not show a statistically significant difference compared to both Azamax® and Roundup®. Although bees fed with the honey+Karate® solution exhibited a response similar to the control (0.591 ± 0.025 µM of NO2), it did not significantly differ from the treatments with Roundup® and Limonene, resulting in an increase in NO2 levels in the samples (Figure 3).
Figure 3. Mean (+SE) of nitric oxide level dosage (μM of NO2) in Apis mellifera bees submitted to LC50 of the compound Limonene, the insecticide Karate®, the herbicide Roundup®, and to concentration of 250 µL/100 mL of Azamax®. Columns with the same letter do not differ by the Tukey HSD test at 5% probability
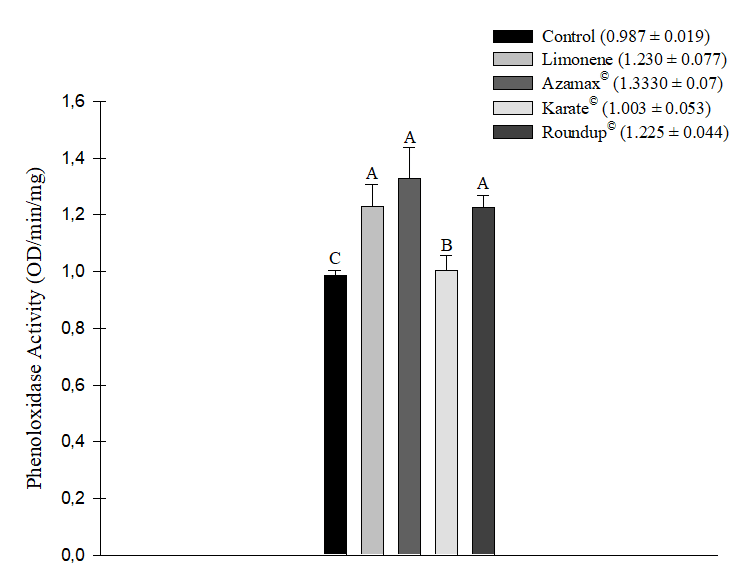
3.4 PHENOLOXIDASE ACTIVITY
All evaluated xenobiotics led to an increase in phenoloxidase enzyme activity after 24 hours of exposure. The most significant increases were observed in the treatments with the biopesticide Azamax® (1.333 ± 0.107 OD/min/mg), followed by the compound Limonene (1.230 ± 0.077 OD/min/mg) and the herbicide Roundup® (1.225 ± 0.044 OD/min/mg), which did not significantly differ from each other. Once again, the Karate® insecticide (1.003 ± 0.053 OD/min/mg) did not exhibit a statistically significant difference from the control group (0.987 ± 0.019 OD/min/mg) (Figure 4).
Figure 4. Phenoloxidase enzyme activity (OD/min/mg) in Apis mellifera bees subjected to LC50 of the compound Limonene, the insecticide Karate®, the herbicide Roundup®, and to concentration of 250 µL/100 mL of Azamax®. Columns with the same letter do not differ by the non-parametric Kruskal-Wallis test at 5% probability
3.5 OXIDATIVE STRESS
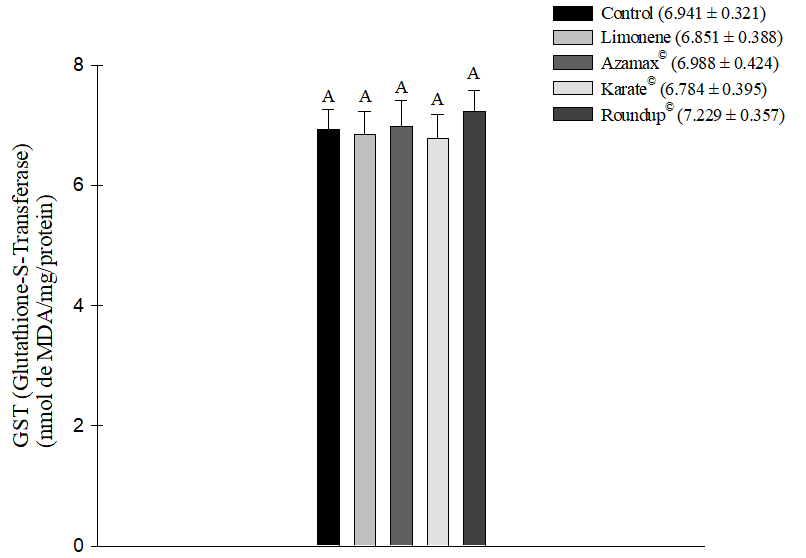
All chemical agents tested, except for Limonene, demonstrated the ability to induce an increase in oxidative stress measured by TBARS, indicating cellular lipid peroxidation in the evaluated bee samples. The herbicide Roundup® (1.838 ± 0.205 nmol of MDA/mg/protein) was particularly noteworthy, being the product that induced the most significant oxidative stress in the cells, followed by Azamax® (1.280 ± 0.070 nmol of MDA/mg/protein) and Karate® (1.214 ± 0.055 nmol MDA/mg/protein). In contrast, the compound Limonene (0.914 ± 0.096 nmol of MDA/mg/protein) did not show a statistically significant difference in comparison to the control group (0.782 ± 0.109 nmol of MDA/mg/protein). Consequently, this substance did not promote this type of stress after 24 hours of exposure (Figure 5). Additionally, during this same evaluation period, there were no differences in the measurement levels of Glutathione-S-Transferase (GSH) (Figure 6).
Figure 5. Oxidative stress from the measurement of thiobarbituric acid (TBARS) as an indicator of lipid peroxidation (nmol of MDA/mg/protein) in Apis mellifera subjected to LC50 of the compound Limonene, the insecticide Karate®, the herbicide Roundup®, and to concentration of 250 µL/100 mL of Azamax®. Columns with the same letter do not differ by the non-parametric Kruskal-Wallis test at 5% probability
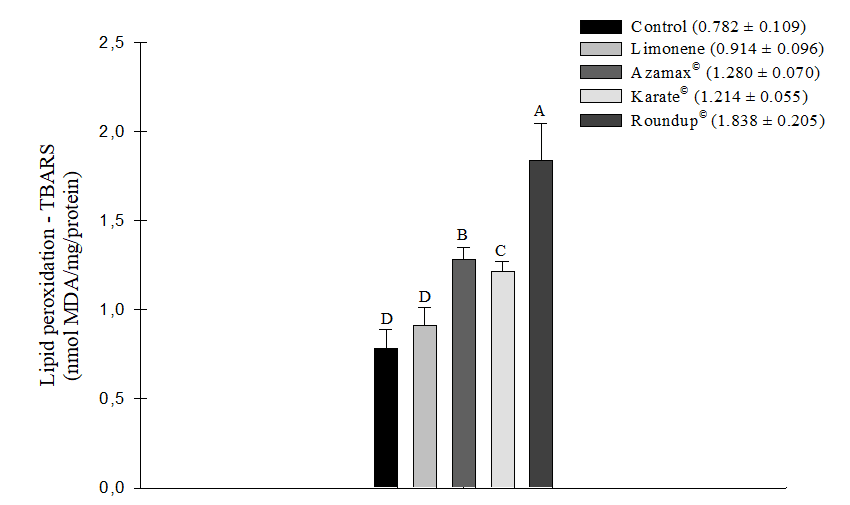
Figure 6. Oxidative stress from the measurement of Glutathione-S-Transferase (GSH) in Apis mellifera subjected to LC50 of the compound Limonene, the insecticide Karate®, the herbicide Roundup®, and to concentration of 250 µL/100 mL of Azamax®. Columns with the same letter do not differ by Tukey’s test at 5% probability, for GSH data
4. DISCUSSION
The histopathological and immunological changes observed in treated bees suggest that the tested substances were highly harmful to these organisms and did not allow them sufficient time to activate regeneration mechanisms (Illa-Bochaca & Montuenga, 2006; Caccia et al., 2019). Considering that, in general, natural products are considered selective, this finding is significant. Natural compounds are primarily assessed for their acute toxicity to beneficial insects (Sabahi et al., 2022; Cappa et al., 2022). However, there is limited information on how these compounds affect bees at the cellular level, which has severe implications for their physiology and health.
Braga et al. (2020) also identified the absence of apoptosis in the midgut of 5th instar nymphs of the predatory bug Podisus nigrispinus (Dallas), which had been fed Alabama argillacea (Hübner) caterpillars treated with a Lethal Dose (LD50) of 2.50 and 2.82 mg/g insect, respectively, of the oils of Mentha spicata L. and Melaleuca alternifolia Cheel. However, in nymphs treated with M. alternifolia, histopathological changes such as columnar cell elongation and cell lysis, similar to the symptoms of necrosis, were observed. Necrosis is characterized by the dilation of cytoplasm, mitochondria, and endoplasmic reticulum, leading to cell rupture and, consequently, the extravasation of cellular content (Sun et al., 2021). Both oils consist solely of terpenes, a chemical class to which Limonene and azadirachtin belong. Therefore, epithelial tissue necrosis may be a mechanism of action of these compounds on insects.
Unlike the other analyzed substances, Limonene does not have the oxygen molecule in its composition, which is capable of triggering free radicals harmful to the body, making the individual susceptible to oxidative stress (Kumar et al., 2022). Furthermore, there is already a report that monoterpenes may have antioxidant properties (Yu et al., 2017). Thus, oxidative stress was not expected to be one of the ways in which Limonene acts on honeybees’ physiology.
Limonene’s mode of action is associated with the neuromuscular inhibition of acetylcholinesterase (AChE), leading to AChE accumulation, which generates neuronal excitation and insect death (García et al., 2005; Zarrad et al., 2017; Gadelhaq et al., 2023). Furthermore, in A. mellifera, effects on intestinal cells were observed, resulting from the necrotic lesions observed in the epithelium.
The oxidative stress observed in the other treatments indicates the occurrence of lipid peroxidation in honeybees, which, as a consequence, leads to alterations in the fundamental functions of the cell membrane (Barbosa et al., 2010). Studies have related the apoptotic process as one of the cellular responses to this stress (Li & Wogan, 2005; Kim & Lee, 2020). However, it may also be the cause of triggering epithelial necrosis in the tested A. mellifera population, with this effect being more pronounced in samples treated with glyphosate.
Indeed, studies on this herbicide have shown its impact on the survival and health of various bee species, whether larvae or adults, whether acutely or chronically exposed. This exposure leads to changes in molecular, cellular, histological, and biological systems (Battisti et al., 2023). An important aspect reported by Liao et al. (2017) is that a solution containing water, sugar, and glyphosate (10 ppb) was found to be more attractive to bees when compared to a solution without the herbicide. This raises concerns about the potential contamination of these organisms through nectar in their natural environment.
The midgut constitutes one of the first defense barriers of insects against pathogenic organisms and xenobiotics (Schmid-Hempel, 2005). The impairment of this function is confirmed by the activation of the phenoloxidase cascade and alterations in NO2 levels, indicating the involvement of humoral defense mechanisms in the bees’ cells when exposed to synthetic and natural pesticides.
The low activity of the enzyme phenoloxidase and the reduced level of NO2 in the tests with Karate®, was also observed by Silva et al. (2020) in bugs of the species Podisus nigrispinus (Dallas), submitted to another pyrethroid, deltamethrin, both under the evaluation time of 24 hours. However, after 72 hours of exposure, these authors found an increase in phenoloxidase activity, simultaneously with a rise in total protein levels in P. nigrispinus. Even so, the analysis of apoptosis by the Tunel test, in the case of the bee population sampled in this research, shows cellular necrosis. In addition, the insecticide Karate® and the other compounds studied here caused irreversible damage, as they could not be accompanied by cell repair, consequently leading to the death of the organism (Miller & Zachary, 2017).
Previous studies have also demonstrated the repellency of azadirachtin, the active ingredient in Azamax®, to other bee species (Zhao et al., 2022) and various insects (Ikeura et al., 2013; Andrade et al., 2013). In the case of A. mellifera, the repellent effect of Azamax® was observed at concentrations above 250 µL/100 mL. Therefore, there is potential for this insecticide to be used in Integrated Pest Management (IPM) programs to preserve the abundance of these pollinators in agroecosystems. This is because the mode of action of azadirachtin is associated with increased contamination through ingestion (Martinez & Van Emden, 2001), and in the field, the likelihood of contact with this substance decreases. However, due to Azamax®‘s strong deleterious effects on the digestive and immune systems of bees, it is crucial to take care to ensure that its spraying does not coincide with the foraging time of A. mellifera.
The exposure of this A. mellifera population to the natural insecticide Azamax® and Limonene resulted in responses analogous to those induced by the synthetic pesticides Karate® and Roundup®. These responses included the necrosis of epithelial tissue and the impairment of membrane functions. Despite the supposed safety of Limonene and Azamax® for non-target organisms, they were the substances that most significantly increased nitric oxide levels and phenoloxidase enzyme activity in bees. However, Roundup® was the treatment that caused the most oxidative stress, and like Karate®, it has the aggravating factor of leaving residues in hive substrates. As a result, bees are also affected by chronic exposure to synthetic chemicals.
5. CONCLUSION
Although the natural insecticides tested here are considered safe for non-target organisms, this research has shown that caution is warranted regarding their use in agroecosystems. They were capable of causing deleterious effects on the immune system of bees comparable to synthetic pesticides. Therefore, more research is needed to understand how natural products interfere with the physiology of bees, in order to ensure that botanical insecticides used in agriculture are truly selective and a safe alternative to synthetic pesticides.
ACKNOWLEDGMENTS
This work was supported in part by the FACEPE (Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco) under Grant: R$ 36,000.
CONFLICT OF INTERESTS
There is no conflict of interest on the part of the authors.
REFERENCES
ALMEHMADI, Roqaiah Mohammad. Larvicidal Histopathological and Ultra-structure Studies of Matrichiaria chamomella Extracts Against the Rift Valley Fever Mosquito Culex quinquefascia tus (Culicidaee: Diptera). Journal of Entomology-Academic Journals Inc., v. 8, n. 1, 2011.
ANDRADE, Lígia Helena de et al. Repellent effect of azadirachtin and essential oils on Aphis gossypii Glover (Hemiptera: Aphididae) in cotton plants. Revista Ciência Agronômica, v. 44, p. 628-634, 2013.
ASHE, Paula C.; BERRY, Mark D. Apoptotic signaling cascades. Progress in Neuro-Psychopharmacology and Biological Psychiatry, v. 27, n. 2, p. 199-214, 2003.
BARBOSA, Kiriaque Barra Ferreira et al. Oxidative stress: concept, implications and modulating factors. Rev Nutr, v. 23, n. 4, p. 629-43, 2010.
BARTLING, Merle T. et al. Exposure to low doses of pesticides induces an immune response and the production of nitric oxide in honeybees. Scientific Reports, v. 11, n. 1, p. 6819, 2021.
BATTISTI, Lucas et al. Review on the sublethal effects of pure and formulated glyphosate on bees: Emphasis on social bees. Journal of Applied Entomology, v. 147, n. 1, p. 1-18, 2023.
BRAGA, Valeska Andrea Ático et al. Effect of essential oils of Mentha spicata L. and Melaleuca alternifolia Cheel on the midgut of Podisus nigrispinus (Dallas)(Hemiptera: Pentatomidae). Acta Histochemica, v. 122, n. 3, p. 151529, 2020.
CACCIA, Silvia; CASARTELLI, Morena; TETTAMANTI, Gianluca. The amazing complexity of insect midgut cells: types, peculiarities, and functions. Cell and Tissue research, v. 377, p. 505-525, 2019.
CAPPA, Federico; BARACCHI, David; CERVO, Rita. Biopesticides and insect pollinators: Detrimental effects, outdated guidelines, and future directions. Science of the Total Environment, v. 837, p. 155714, 2022.
COSTA, Hilton Nobre et al. LAMBDA-CYHALOTHRIN PROMOTES OXIDATIVE STRESS AND PATHOLOGICAL CHANGES IN THE MIDGUT AND GONADS OF COTTON BOLL WEEVIL. International Journal of Biological and Natural Sciences, ISSN 2764-1813 v. 2, n. 2, 2022.
CRUZ, Glaucilane S. et al. Effect of trans-anethole, limonene and your combination in nutritional components and their reflection on reproductive parameters and testicular apoptosis in Spodoptera frugiperda (Lepidoptera: Noctuidae). Chemico-Biological Interactions, v. 263, p. 74-80, 2017.
DECOURTYE, A. et al. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Archives of environmental contamination and toxicology, v. 48, p. 242-250, 2005.
EARDLEY, C. et al. Background to pollinators, pollination and food production. The Assessment Report on Pollinators, Pollination and Food Production; Ngo, HT, Eds, p. 1-25, 2016.
FARALDO, Ana Carolina et al. Nitric oxide production in blowfly hemolymph after yeast inoculation. Nitric Oxide, v. 13, n. 4, p. 240-246, 2005.
FARALDO, Ana Carolina et al. Prophenoloxidase activity in blowfly hemolymph after yeast inoculation. Resumos, 2006.
GADELHAQ, Sahar M. et al. D‐limonene nanoemulsion: lousicidal activity, stability, and effect on the cuticle of Columbicola columbae. Medical and Veterinary Entomology, v. 37, n. 1, p. 63-75, 2023.
GARCÍA, Matías et al. Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Management Science: formerly Pesticide Science, v. 61, n. 6, p. 612-618, 2005.
GAVRIELI, Yael; SHERMAN, Yoav; BEN-SASSON, Shmuel A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of cell biology, v. 119, n. 3, p. 493-501, 1992.
GIANNINI, Tereza C. et al. The dependence of crops for pollinators and the economic value of pollination in Brazil. Journal of economic entomology, v. 108, n. 3, p. 849-857, 2015.
GILL, Richard J.; RAINE, Nigel E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Functional Ecology, v. 28, n. 6, p. 1459-1471, 2014.
GOULSON, Dave et al. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science, v. 347, n. 6229, p. 1255957, 2015.
GRAYSTOCK, Peter et al. The T rojan hives: pollinator pathogens, imported and distributed in bumblebee colonies. Journal of Applied Ecology, v. 50, n. 5, p. 1207-1215, 2013.
GREEN, Laura C. et al. Nitrate biosynthesis in man. Proceedings of the National Academy of Sciences, v. 78, n. 12, p. 7764-7768, 1981.
GREGORC, Ales; ELLIS, James D. Cell death localization in situ in laboratory reared honey bee (Apis mellifera L.) larvae treated with pesticides. Pesticide biochemistry and physiology, v. 99, n. 2, p. 200-207, 2011.
HERBERT, Lucila T. et al. Effects of field-realistic doses of glyphosate on honeybee appetitive behaviour. Journal of experimental biology, v. 217, n. 19, p. 3457-3464, 2014.
IKEURA, Hiromi; SAKURA, Akio; TAMAKI, Masahiko. Repellent effect of neem against the cabbage armyworm on leaf vegetables. Journal of Agriculture and Sustainability, v. 4, n. 1, 2013.
ILLA-BOCHACA, Irineu; MONTUENGA, Luis M. The regenerative nidi of the locust midgut as a model to study epithelial cell differentiation from stem cells. Journal of experimental biology, v. 209, n. 11, p. 2215-2223, 2006.
ISMAN, Murray B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol., v. 51, p. 45-66, 2006.
ISMAN, Murray B. Botanical insecticides in the twenty-first century—fulfilling their promise?. Annual Review of Entomology, v. 65, p. 233-249, 2020.
JUNQUEIRA, Luiz Carlos Uchoa; JUNQUEIRA, L. M. M. S. Técnicas básicas de citologia e histologia. São Paulo: Santos, v. 8, p. 3, 1983.
KIM, Heesu; LEE, Dong Gun. Nitric oxide–inducing Genistein elicits apoptosis-like death via an intense SOS response in Escherichia coli. Applied Microbiology and Biotechnology, v. 104, p. 10711-10724, 2020.
KLEIN, Alexandra-Maria et al. Importance of pollinators in changing landscapes for world crops. Proceedings of the royal society B: biological sciences, v. 274, n. 1608, p. 303-313, 2007.
KUMAR, Debaditya et al. Oxidative stress and apoptosis in Asian honey bees (A. cerana) exposed to multiple pesticides in intensive agricultural landscape. Apidologie, v. 53, n. 2, p. 25, 2022.
LI, Chun-Qi; WOGAN, Gerald N. Nitric oxide as a modulator of apoptosis. Cancer letters, v. 226, n. 1, p. 1-15, 2005.
LIAO, Ling-Hsiu; WU, Wen-Yen; BERENBAUM, May R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Scientific Reports, v. 7, n. 1, p. 15924, 2017.
LOSA, Marco et al. Determination of the proliferation and apoptotic index in adrenocorticotropin-secreting pituitary tumors: comparison between micro-and macroadenomas. The American journal of pathology, v. 156, n. 1, p. 245-251, 2000.
LOWRY, OliverH et al. Protein measurement with the Folin phenol reagent. Journal of biological chemistry, v. 193, n. 1, p. 265-275, 1951.
MARTINEZ, Sueli S.; VAN EMDEN, Helmut F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval)(Lepidoptera: Noctuidae) caused by azadirachtin. Neotropical Entomology, v. 30, p. 113-125, 2001.
MAULIK, Nilanjana; YOSHIDA, Tetsuya; DAS, Dipak K. Oxidative stress developed during the reperfusion of ischemic myocardium induces apoptosis. Free Radical Biology and Medicine, v. 24, n. 5, p. 869-875, 1998.
MICHENER, C. D. The Bees of the World Johns Hopkins University Press. Baltimore, Md, USA, 2007.
MILLER, Margaret A.; ZACHARY, James F. Mechanisms and morphology of cellular injury, adaptation, and death. Pathologic basis of veterinary disease, p. 2, 2017.
MULLIN, Christopher A. et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PloS one, v. 5, n. 3, p. e9754, 2010.
NEGREIRO, Maria Cláudia Cordeiro; DE ANDRADE, Fábio Goulart; FALLEIROS, Ângela Maria Ferreira. Sistema imunológico de defesa em insetos: uma abordagem em lagartas da soja, Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae), resistentes ao AgMNPV. Semina: Ciências Agrárias, v. 25, n. 4, p. 299-313, 2004.
OLIVEIRA, Fernanda M. et al. Histological, histochemical and energy disorders caused by R-limonene on Aedes aegypti L. larvae (Diptera: Culicidae). Acta Tropica, v. 221, p. 105987, 2021.
OHKAWA, Hiroshi; OHISHI, Nobuko; YAGI, Kunio. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical biochemistry, v. 95, n. 2, p. 351-358, 1979.
POTTS, Simon G. et al. Global pollinator declines: trends, impacts and drivers. Trends in ecology & evolution, v. 25, n. 6, p. 345-353, 2010.
POTTS, Simon G. et al. The assessment report on pollinators, pollination and food production: summary for policymakers. Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 2016.
RUIZ-TOLEDO, Jovani; SÁNCHEZ-GUILLÉN, Daniel. Effect of the concentration of glyphosate present in body waters near transgenic soybean fields on the honeybee Apis mellifera, and the stingless bee Tetragonisca angustula. Acta zoológica mexicana, v. 30, n. 2, p. 408-413, 2014.
SABAHI, Qodratollah; KELLY, Paul Gordon; GUZMAN‐NOVOA, Ernesto. Carvone and citral, two promising compounds for controlling the honey bee ectoparasitic mite, Varroa destructor. Journal of Applied Entomology, v. 146, n. 8, p. 1003-1010, 2022.
SEDLAK, Jozef; LINDSAY, Raymond H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical biochemistry, v. 25, p. 192-205, 1968.
SILVA, Cristiane TS et al. Immune and nutritional responses of Podisus nigrispinus (Hemiptera: Pentatomidae) nymphs sprayed with azadirachtin. Austral Entomology, v. 59, n. 1, p. 215-224, 2020.
SOLOMON, Robert W. Free and open source software for the manipulation of digital images. American Journal of Roentgenology, v. 192, n. 6, p. W330-W334, 2009.
SOUZA, Catiane Oliveira et al. Toxicology, histophysiological and nutritional changes in Apis mellifera (Hymenoptera: Apidae) submitted to limonene and natural pesticides in comparison to synthetic pesticides. Journal of Apicultural Research, p. 1-12, 2023.
SUN, Zhipeng et al. Rotenone-induced necrosis in insect cells via the cytoplasmic membrane damage and mitochondrial dysfunction. Pesticide Biochemistry and Physiology, v. 173, p. 104801, 2021.
SCHMID-HEMPEL, Paul. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol., v. 50, p. 529-551, 2005.
WU, Xing et al. Determination of the apoptotic index in osteosarcoma tissue and its relationship with patients prognosis. Cancer Cell International, v. 13, p. 1-4, 2013.
YU, Lihua; YAN, Jing; SUN, Zhiguang. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Molecular medicine reports, v. 15, n. 4, p. 2339-2346, 2017.
ZARRAD, Khaoula et al. Chemical composition and insecticidal effects of Citrus aurantium of essential oil and its powdery formulation against Tuta absoluta. Tunis. J. Plant Prot, v. 12, p. 83-94, 2017.
ZATTARA, Eduardo E.; AIZEN, Marcelo A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth, v. 4, n. 1, p. 114-123, 2021.
ZHAO, Kunyu et al. Effects of sublethal azadirachtin on the immune response and midgut microbiome of Apis cerana cerana (Hymenoptera: Apidae). Ecotoxicology and Environmental Safety, v. 229, p. 113089, 2022.
[1] Master in Entomology – UFRPE. ORCID: 0009-0009-3350-2334. Currículo Lattes: http://lattes.cnpq.br/3858015372572475.
[2] PhD in Nuclear Technology – USP. ORCID: 0000-0001-9533-5476. Currículo Lattes: http://lattes.cnpq.br/4292195468804301.
[3] Advisor. PhD in Chemistry – UFRPE. ORCID: 0000-0001-8508-1230. Currículo Lattes: http://lattes.cnpq.br/5615678215435460.
[4] Master Entomology – UFRPE. ORCID: 0000-0002-6223-7113. Currículo Lattes: http://lattes.cnpq.br/5156282820589894.
[5] PhD in the Postgraduate Program in Entomology – UFRPE. ORCID: 0000-0001-6012-1945. Currículo Lattes: http://lattes.cnpq.br/3795270436231657.
[6] PhD in Entomology – UFRPE. ORCID: 0000-0002-4270-1476. Currículo Lattes: http://lattes.cnpq.br/8583960034442631.
[7] PhD in Chemistry – USP. ORCID: 0000-0002-7597-6775. Currículo Lattes: http://lattes.cnpq.br/6957579091162269.
[8] Doctor in Biochemistry. ORCID: 0000-0002-6041-1720. Currículo Lattes: http://lattes.cnpq.br/4162837199448101.
[9] PhD in Animal Production from the Faculty of Agricultural Sciences and Veterinarians, Jaboticabal, UNESP. ORCID: 0000-0002-3488-4778. Currículo Lattes: http://lattes.cnpq.br/3266223126925865.
[10] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: 0000-0003-1493-7964. Currículo Lattes: http://lattes.cnpq.br/9044747136928972.
[11] PhD in morphology – UNIFESP. ORCID: 0000-0001-5940-9220. Currículo Lattes: http://lattes.cnpq.br/1539131079574469.
Material recebido: 05 de dezembro de 2023.
Material aprovado pelos pares: 30 de janeiro de 2024.
Material editado aprovado pelos autores: 16 de fevereiro de 2024.