ORIGINAL ARTICLE
SANTOS, Yasmim Barbosa dos [1], SILVA, Maria Vanessa da [2], NASCIMENTO, Bruno José do [3], SANTOS, Anthony Marcos Gomes dos [4], FRANÇA, Marcelle Mariana Sales de [5], MEDINA, Vanessa Bischoff [6], SOARES, Anísio Francisco [7], TEIXEIRA, Álvaro Aguiar Coelho [8], TEIXEIRA, Valeria Wanderley [9]
SANTOS, Yasmim Barbosa dos et al. Immunomodulatory effect of melatonin on the offspring of rats exposed to alcohol during gestation and lactation. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 09, Ed. 09, Vol. 01, pp. 127-150. September 2024. ISSN: 2448-0959, Acess link: https://www.nucleodoconhecimento.com.br/biology/rats-exposed-to-alcohol, DOI: 10.32749/nucleodoconhecimento.com.br/biology/rats-exposed-to-alcohol
ABSTRACT
This study investigated the immunomodulatory effect of melatonin on the thymus and spleen of offspring from dams exposed to alcohol consumption during pregnancy and lactation. Alcohol (3 g/kg) and melatonin (0.8 mg/kg) were administered from pregnancy confirmation until the end of lactation. For the experiment, three groups of Wistar rats were formed: Control – Offspring not subjected to alcohol; Alcohol – Offspring exposed to alcohol; Alcohol + Melatonin – Offspring exposed to both alcohol and melatonin. Histological and morphometric analyzes showed that the spleen of the alcohol group showed an increase in the number of hematopoietic cells and a significant reduction in the white pulp, as well as the thymus of this group revealed a reduction in the thymic area, these changes were not observed in the alcohol+mel groups and control. The organosomatic index of these organs also revealed a significant reduction only in the alcohol group. In immunohistochemistry, the alcohol+mel and control groups exhibited weak IL-1β staining and a low apoptotic index in the thymus and spleen when compared to the alcohol group. Thus, we conclude that the administration of melatonin simultaneously with alcohol consumption during pregnancy and lactation can protect the thymus and spleen of the offspring of alcoholic mothers from damage caused by increased oxidative stress.
Key Words: Alcoholism rats, Fetus, Immunological, Pineal, Pregnancy.
1. INTRODUCTION
Alcohol is considered a teratogenic substance as it can cause a variety of congenital disorders. Therefore, ethanol consumption during pregnancy is risky for both the mother and the developing fetus (Gupta; Gupta; Shirasaka, 2016). In Brazil, approximately 10 to 40% of pregnant women use ethanol during pregnancy (Moraes; Reichenheim, 2007; Costa et al., 2014; Baptista et al., 2017).
Factors such as enzyme deficiency and inefficiency, as well as vasoconstriction of the placenta and umbilical cord, contribute to the reduced elimination of alcohol, resulting in its prolonged effect on the fetus (Burd et al., 2007). It is also possible through lactation for exposure to cause damage to the immune and nervous systems of the newborn (Haastrup; Pottegård; Damkier, 2014; Deak et al., 2022).
The increase in Reactive Oxygen Species (ROS) and the decrease in antioxidants caused by ethanol metabolism can result in cellular damage, mitochondrial dysfunction, protein and DNA damage, as well as induce apoptosis and lipid peroxidation (Cohen-Kerem; Koren, 2003; Rumgay et al., 2021). Alcohol is also known to be an immunomodulator, causing alterations in cytokine levels, action zone and metabolism (Pascual et al., 2016; Chen et al 2017; Adams et al., 2020).
Considering this, studies indicate that ethanol consumption can lead to losses due to apoptosis or reduction of cells during intrauterine development in the thymus and spleen, potentially impairing the activity and morphology of these organs (Corazza et al., 1997; Veiga et al., 2007). Chronic alcohol consumption results in immunosuppression, compromising cellular and humoral immunity, consequently making alcoholics more susceptible to acquiring infections (Budec et al., 1992; Ince; Curabeyoğlu; Akyol, 2019; Molina et al., 2023).
Studies have utilized antioxidants such as melatonin to reduce damage caused by free radicals. This indolamine is effective in combating oxidative stress by directly acting on reactive oxygen species, stimulating the activity of antioxidant enzymes, and inhibiting the action of pro-oxidants (Reiter et al., 2016; Kurhaluk, 2021). Melatonin is capable of easily crossing the placenta and reaching the fetal circulation; other scientific evidence also suggests the possibility of transfer to the newborn through lactation (Molad et al., 2019; Joseph et al., 2024).
Considering the impairment of the immune system by ethanol, it is relevant to investigate the protective role of melatonin against these injuries in the offspring. Therefore, the aim of this study was to evaluate the effects of exogenous melatonin on the thymus and spleen of offspring whose dams were subjected to chronic alcohol consumption during pregnancy and lactation.
2. MATERIALS AND METHODS
The experiment was conducted at the Laboratory of Morphological Studies of Vertebrates and Invertebrates (LABEMOVI) at the Federal Rural University of Pernambuco (UFRPE), with approval from the Institutional Ethics Committee (CEUA) under number 3325300821. For the completion of this research, thirty female albino rats (Rattus norvegicus albinus) from the Wistar strain, weighing approximately 200 ± 30g, were used, sourced from the Animal Morphology and Physiology Department’s vivarium.
The animals were housed in cages under standard conditions of 22 ± 1°C and artificial lighting provided by fluorescent lamps. The alcohol and melatonin treatments were initiated on the first day of confirmed pregnancy and administered until the end of lactation. After mating and confirmation of pregnancy, the animals were divided into the following experimental groups: Control – Offspring that were not exposed to alcohol; Alcohol – Offspring exposed to alcohol through their mothers during gestation and lactation; Alcohol + Melatonin – Offspring exposed to alcohol and melatonin through their mothers during gestation and lactation. This study is a continuation of the paper published on August 28, 2023. As such, both studies are part of the same experiment.
2.1 COLPOCYTOLOGICAL EXAMINATION
In the early morning, for the collection of vaginal material from the rats, cotton swabs moistened with physiological saline were used and inserted into the animal’s vagina with rotational movements. Then, the collected material was transferred to histological slides, also by rotating the swab on the slides. The slides were stained with Toluidine Blue for 3 seconds and observed under a light microscope to analyze the presence or absence of spermatozoa. If confirmed, this day was considered the first day of pregnancy.
2.2 ETHANOL AND MELATONIN ADMINISTRATION
Ethanol was administered intragastrically at a dosage of 3 g/kg of ethyl alcohol to the pregnant and lactating rats (Varlinskaya; Spear; Spear, 2001; Araújo-Filho et al., 2007; Veiga et al., 2007; Scheidt et al., 2015; Marco et al., 2017) diluted in distilled water. Melatonin, N-acetyl-5-methoxytryptamine (Sigma Chemical Co., St. Louis, USA), was administered in daily injections of 0.8 mg/kg throughout gestation. For this purpose, melatonin was dissolved in 0.2 mL of ethanol and diluted in 0.8 mL of 0.9% NaCl. This dose is comparable to the human dosage (9 mg/kg), which was converted based on body surface area (Moustafa et al., 1999; Abd-Allah et al., 2003).
2.3 EUTHANASIA
After the treatment, 12 pups from each group, regardless of sex, were anesthetized at 30 days of age with ketamine hydrochloride (80 mg/kg) and xylazine (6 mg/kg) intramuscularly and euthanized by deepening the anesthesia. The thymus and spleen were removed by opening the abdominal cavity, weighed, and fixed in 10% buffered formalin solution for 48 hours.
2.4 HISTOPATHOLOGY
After collection and fixation, the material underwent routine histological processing for embedding in paraffin. Histological sections were cut at a thickness of 5 μm using a Minot-type microtome (Leica RM 2035). On the slides, the material underwent the hematoxylin-eosin (H&E) staining procedure and was analyzed under a light microscope, OLYMPUS BX-49, and photographed using an OLYMPUS BX-50 microscope.
2.5 MORPHOMETRIC ANALYSIS
For the morphometry of the pups’ spleen and thymus, images were captured using a Sony® video camera attached to an Olympus® Bx50 microscope. These images were subjected to morphometry analysis using the Line Morphometry application, calibrated in micrometers, associated with the ImageLab 2000 for Windows program. In the spleen, 10 splenic corpuscles from each experimental group were measured to determine the following characteristics in micrometers: area, maximum diameter, and minimum diameter of the white pulp (Golalipour et al., 2008). The percentage of hematopoietic cells was determined using a reticle containing 100 points, coupled with a 10x eyepiece and analyzed with a 40x objective (Weibel; Kistler; Scherle, 1966). The points that coincided with the hematopoietic cells were counted. In the thymus, five slides per group were used, and 10 thymic lobes were measured to determine the thymic area, cortical area, and medullary area (Alves et al., 2006). All measurements were performed using Image J software.
2.6 IMMUNOHISTOCHEMICAL ANALYSIS OF IL-1Β
For interleukin, the slides were subjected to antigen retrieval in citrate buffer (pH 8.0) in the microwave for 5 minutes. Endogenous peroxidase was inhibited using a solution of hydrogen peroxide (3%) in methanol. Non-specific antigen-antibody reaction was blocked by incubating the slides in 5% PBS and Bovine Serum Albumin (BSA) for one hour. Subsequently, the slides were treated with primary IL-1β antibodies (sc-E7-2-hIL1β – Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted at a ratio of 1:50 in 1% PBS/BSA. The slides were then treated with Histofine® (Cod. 414191F – Nichirei Biosciences, Tokyo, Japan) for 30 minutes. The antigen-antibody reaction in the sections was visualized by applying the chromogen 3,3′-diaminobenzidine for 4 minutes and counterstained with hematoxylin (Oberholzer et al., 1996; Lee et al., 2001). Images were captured using a Sony® video camera attached to an Olympus® Bx50 microscope and subjected to the Gimp 2.0 application for pixel quantification using RGB (Red-Green-Blue) histogram in the selected areas. The pixel tones in the image range from 0 to 255, where tone 0 represents absolute darkness (lowest luminescence), and tone 255 represents absolute white (highest luminescence). Two slides from each group were used, and four fields/slide were photographed for the measurements.
2.7 APOPTOTIC INDEX
For apoptosis, the TUNEL method was used. The sections were initially deparaffinized and hydrated, and then incubated in PBS for 5 minutes at room temperature. Proteinase K was applied to the slides for 15 minutes. The slides were washed in distilled water and incubated in hydrogen peroxide for 5 minutes at room temperature. The sections were washed in PBS and incubated in equilibrium buffer for 60 minutes at 4 °C. The sections were then incubated in TdT at 37 °C for 1 hour in a humidified chamber. The “stop” solution was applied for 10 minutes at room temperature, followed by washing the slides in PBS and incubating th+em in anti-digoxigenin. The sections were rinsed with PBS and subjected to the chromogenic substrate diaminobenzidine (DAB, DakoCytomationTM) for ±20 minutes, counterstained with hematoxylin for 20 to 30 seconds. The slides were then washed in running water, dehydrated in increasing concentrations of alcohol, and placed in xylene for mounting and observation under a light microscope. The apoptotic index was determined by counting the percentage of positive cells from at least 500 nuclei subdivided into 10 randomly chosen fields using a 40x objective (Wu et al., 2013).
2.8 ORGANOSOMATIC INDEX (OSI)
To obtain the organosomatic index, the pups and organs were weighed using a precision balance, and the ratio between the organ weight (thymus and spleen) and body weight of each animal was calculated using the following formula: OI: Organosomatic index; OW: Organ weight; BW: Body weight.
2.9 STATISTICAL ANALYSIS
For data comparison, Analysis of Variance (ANOVA) was performed. When significant, it was supplemented with Tukey’s multiple comparisons test and Kramer’s test (P < 0.05).
3. RESULTS
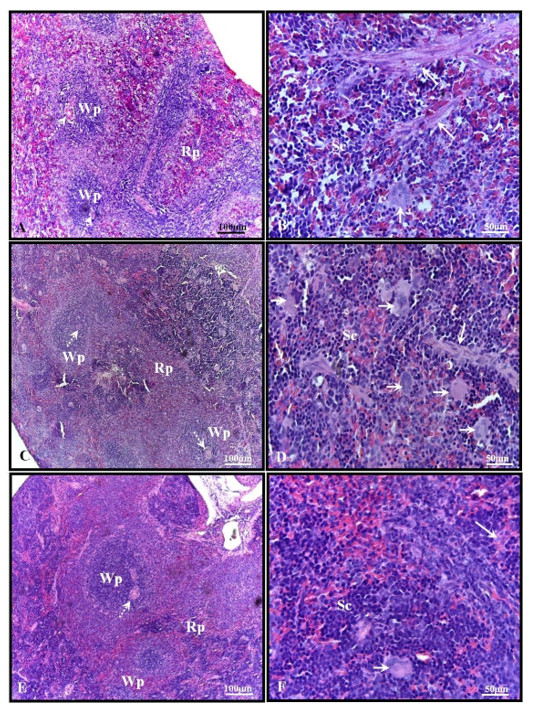
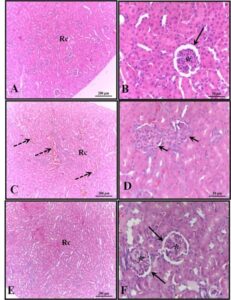
Histological analysis showed that the spleen of 30-day-old pups from the control group exhibited parenchyma composed of white pulp, characterized by lymphatic nodules with arterioles surrounded by red pulp containing trabeculae of dense connective tissue and smooth muscle fibers, splenic cords or Billroth’s cords, sinusoidal capillaries, and few hematopoietic cells (Figures 1A-B). In the spleen of pups from the alcohol group, although similar characteristics to those observed in the control group were found, several hematopoietic cells were present in the red pulp (Figures 1C-D). No significant histological changes were observed in the alcohol + mel group (Figures 1E-F).
Figure 1: Photomicrograph of the spleen of 30-day-old pups from the experimental groups. (A-B) Control: observe the parenchyma composed of white pulp, surrounded by red pulp containing trabeculae of dense connective tissue and smooth muscle fibers, splenic cords, and few hematopoietic cells. (C-D) Alcohol: note the presence of several hematopoietic cells in the red pulp. (E-F) Alcohol + Mel: no significant histological changes were observed. Wp – white pulp; Rp – red pulp; Dotted arrow – arteriole; Short arrow – hematopoietic cells; Long arrow – trabeculae; Sc – splenic cords. H.E.
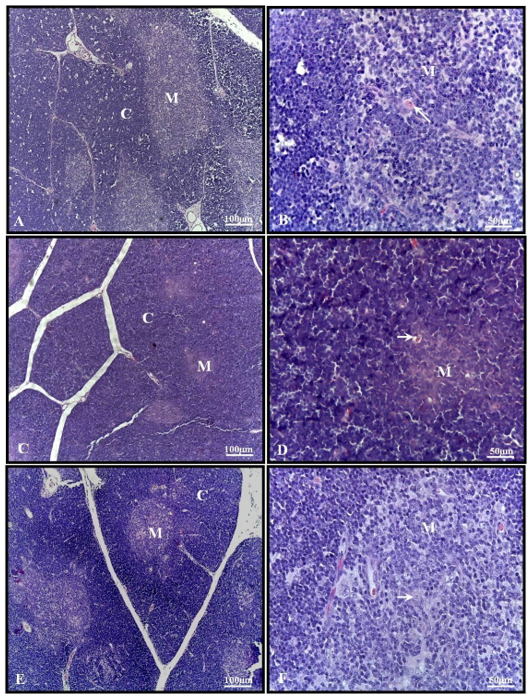
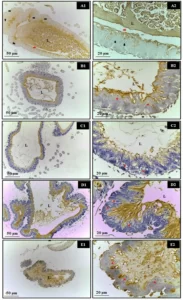
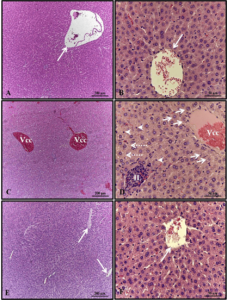
The thymus of 30-day-old pups from the control group presented several lobules containing well-developed cortical and medullary zones, with the presence of Hassall’s corpuscles in the latter (Figures 2A-B). In the alcohol group, the thymus lobules showed a well-developed cortical zone and a less developed medulla, but with Hassall’s corpuscles (Figures 2C-D). The thymus of the alcohol + mel group exhibited histological characteristics like those observed in the control group (Figures 2E-F).
Figure 2: Photomicrograph of the thymus of 30-day-old pups from the experimental groups. (A-B) Control: multiple lobules with well-developed cortical and medullary zones. (C-D) Alcohol: note the well-developed cortical zone and less developed medulla. (E-F) Alcohol + Mel: exhibited histological characteristics similar to the control group. M – medulla; C – cortex; Arrow – Hassall’s corpuscles. H.E.
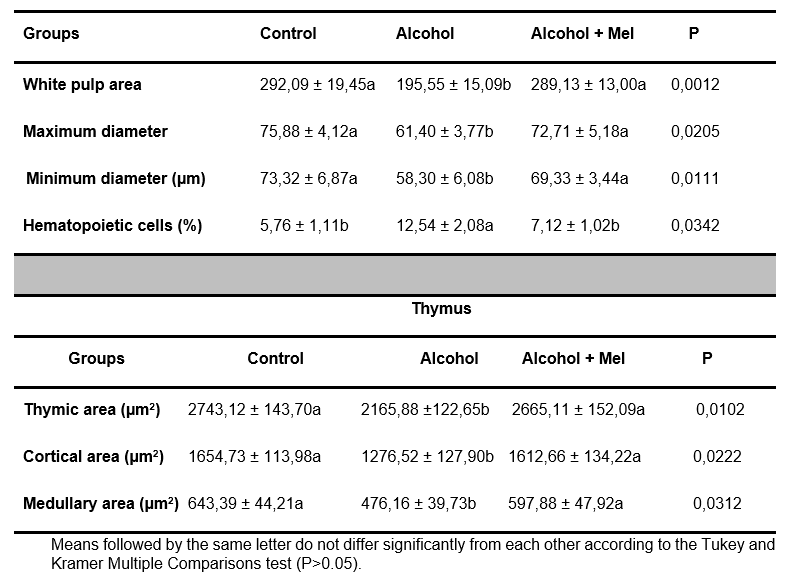
Morphometric analysis of the spleen revealed a significant reduction in the area, major and minor diameter of the white pulp, and a high percentage of hematopoietic cells in the alcohol group pups. Regarding the thymus, these same animals showed a reduction in the cortical and medullary zones, resulting in a reduction in thymic area. These effects were prevented by melatonin administration (Table 1).
Table 1. Mean values of morphometric parameters of the spleen and thymus in 30-day-old pups
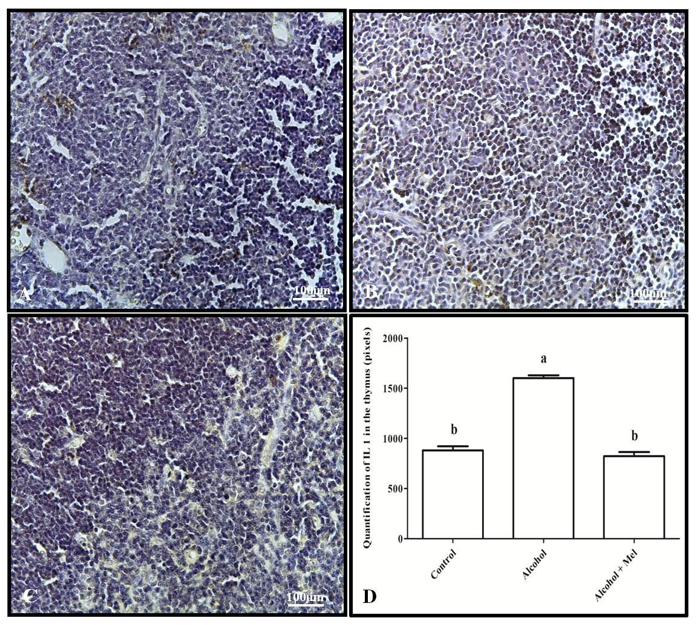
Immunohistochemical analysis of IL-1β showed strong labeling in the spleen and thymus of the alcohol group animals compared to the other experimental groups, which was confirmed by pixel quantification (Figures 3 and 4).
Figure 3: Immunohistochemistry for IL-1 in the spleen of 30-day-old pups from the experimental groups. (A) Control: weak labeling; (B) Alcohol: strong labeling; (C) Alcohol + Mel: weak labeling; and (D) Quantification in pixels. Means followed by the same letters do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P<0.05)
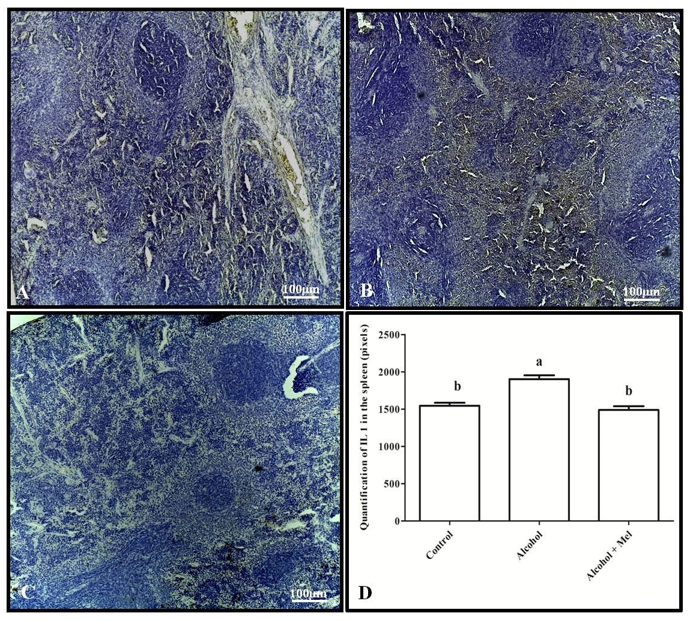
Figure 4: Immunohistochemistry for IL-1 in the thymus of 30-day-old pups from the experimental groups. (A) Control: weak labeling; (B) Alcohol: strong labeling; (C) Alcohol + Mel: weak labeling; and (D) Quantification in pixels. Means followed by the same letters do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P<0.05)
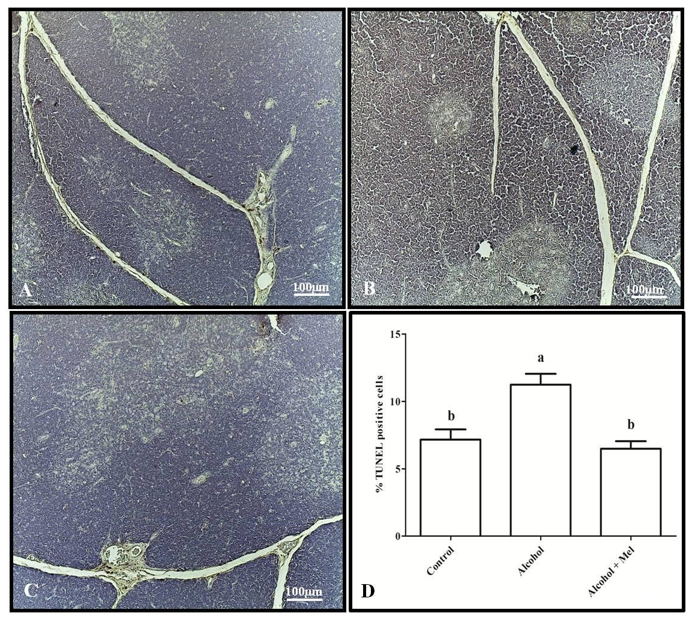
As for the apoptotic index, the alcohol group exhibited the highest indices in both the spleen and thymus compared to the other groups (Figures 5 and 6).
Figure 5: TUNEL assay in the spleen of 30-day-old pups from the experimental groups. (A) Control: lower apoptotic index; (B) Alcohol: higher index; (C) Alcohol + Mel: lower index; and (D) Apoptotic index. Means followed by the same letters do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P<0.05)
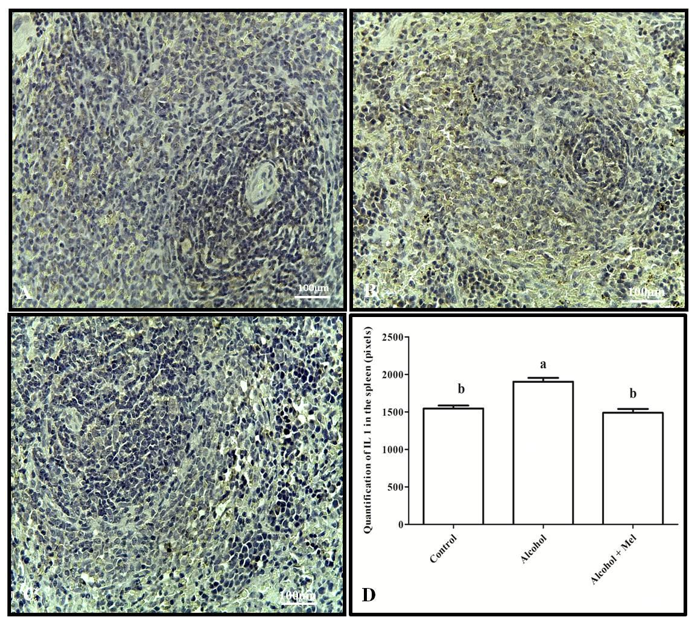
Figure 6: TUNEL assay in the thymus of 30-day-old pups from the experimental groups. (A) Control: lower apoptotic index; (B) Alcohol: higher index; (C) Alcohol + Mel: lower index; and (D) Apoptotic index. Means followed by the same letters do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P<0.05)
Statistical analysis of the organosomatic index of the spleen and thymus revealed a significant reduction in the alcohol group animals compared to the other experimental groups (Figures 7 and 8).
Figure 7: Organosomatic index of the spleen in animals from the experimental groups. Alcohol: Significant reduction in the organosomatic index observed; Control and Alcohol + Mel: No significant difference observed. Means followed by the same letter do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P>0.05)
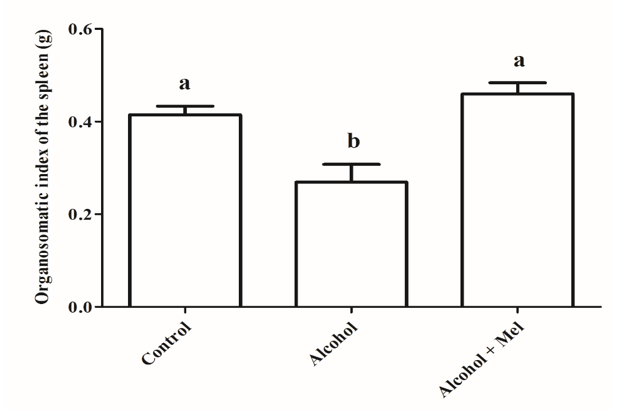
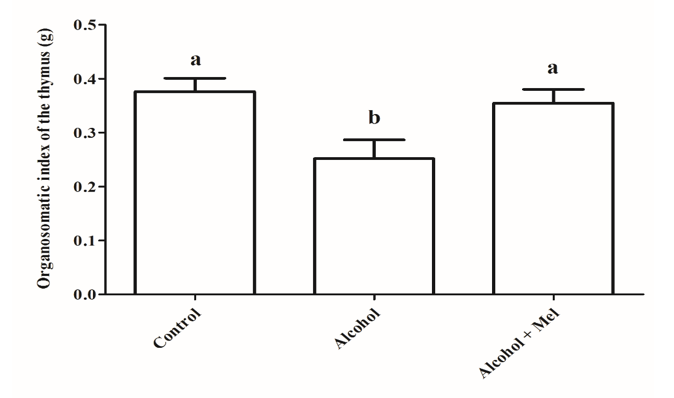
Figure 8: Organosomatic index of the thymus in animals from the experimental groups. Alcohol: Significant reduction in the organosomatic index observed; Control and Alcohol + Mel: No significant difference observed. Means followed by the same letter do not differ significantly from each other according to Tukey and Kramer’s multiple comparison test (P>0.05)
4. DISCUSSION
Alcohol is known as a teratogen responsible for various physical and behavioral alterations in the fetus, resulting from its prolonged action on the fetal organism. These effects can be caused by increased Reactive Oxygen Species (ROS), decreased antioxidant enzymes, cellular damage, and inhibition of important cofactors for fetal development (Gupta; Gupta; Shirasaka, 2016).
In the histopathological and morphometric analyses of the spleen, a high percentage of hematopoietic cells was observed in the alcohol group, which can occur in the spleen when there is a stimulus to the immune response or infections in mammals such as rodents (Johns; Christopher, 2012). According to Kim (2010) and Barbieri et al. (2017), hematopoiesis can occur outside the bone marrow in situations of drug toxicity, inflammation, and repair, as well as in abnormal production of chemokines.
Furthermore, animals in this group showed a significant reduction in the white pulp in the spleen, which is a region rich in B lymphocytes and contains T lymphocytes (Steiniger, 2015). Additionally, the alcohol group exhibited higher apoptotic indices in the spleen compared to the control and alcohol + mel groups. Authors such as Slukvin; Jerrells (1995) and Liu et al. (2016) have reported apoptosis of T and B lymphocytes and a significant decrease in spleen size induced by ethanol. Chronic alcohol consumption can cause increased oxidative stress and various cellular damage, resulting in alterations in the function and death of immune system cells (Chitimus et al., 2020). This is because ethanol promotes the production of reactive oxygen species, which can react and damage proteins, membrane lipids, mitochondria, and DNA. Moreover, alcohol affects the body’s natural defense systems against ROS (Wu; Cederbaum, 2003). Therefore, the results of this study demonstrate that ethanol caused these alterations in the offspring during gestation and lactation.
Similarly, significant reductions in the cortical and medullary areas of the thymus were observed in the morphometric and histological findings of the alcohol group compared to the other groups, resulting in a decrease in thymic area, along with a high apoptotic index. Ethanol is capable of inducing lymphocyte depletion in lymphoid organs through apoptosis and autophagy of cells, such as thymocytes, resulting from oxidative stress and mitochondrial damage (Eid et al., 2000; Kapasi et al., 2003). Studies have reported oxidative damage caused by ethanol in the lymphoid tissue of newborn rats and a significant reduction in thymus weight and cell number in adult rats subjected to chronic alcohol consumption (Wang; Spitzer, 1997; Ince; Curabeyoğlu; Akyol, 2019). Moreover, newborn exposure to alcohol through breast milk also impairs the functioning of the immune system, leading to reduced resistance to infection (Seelig; Steven; Stewart, 1999; Zhu; Seelig, 2000).
These changes observed in the alcohol group were not found in the alcohol + mel group, suggesting the potential therapeutic effects of melatonin on the fetus during gestation and lactation. Authors such as Santos et al., (2023) have observed the attenuation of damage by melatonin in other organs of offspring exposed to alcohol. According to Kelty et al. (2021), despite the various damages related to alcohol consumption during pregnancy, the use of medications to treat pregnant women with alcohol use disorder is still considered rare due to the lack of research and information on the safety of these medications during pregnancy. Melatonin, on the other hand, has low toxicity and can easily cross all morphophysiological barriers, including cell membranes, attenuate oxidative damage, and maintain mitochondrial function. It can directly remove reactive species and stimulate the activity of antioxidant enzymes, thereby reducing cellular and macromolecular damage in various organs (Reiter et al., 2000; Ghosh et al., 2023). These findings support our results, as melatonin administered along with alcohol prevented alterations in histological structures and the increase in apoptotic index in the thymus and spleen.
In the present study, strong IL-1β immunohistochemical staining was observed in the thymus and spleen of the alcohol group. According to Hoek and Pastorino (2002) and Pradier et al. (2018), cytokines are considered essential mediators of immune responses and can undergo alterations with alcohol use. Excessive alcohol consumption promotes the production of pro-inflammatory cytokines, which when released by immune cells can trigger defensive responses in parenchymal cells and apoptosis activation. This may explain the high apoptotic levels observed in the thymus and spleen. IL-1β is among the cytokines predominantly affected in chronic alcoholics and may be elevated in mothers and/or fetuses exposed to ethanol (Ahluwalia et al., 2000; Silva-Gotay et al., 2021). In contrast, the alcohol + melatonin group showed no alterations in the level of IL-1β, confirming the anti-inflammatory effects of melatonin by restricting the upregulation of a significant portion of pro-inflammatory cytokines and acting as an immune system modulator (Reiter et al., 2000).
A significant reduction in the Organosomatic Index (OSI) of the spleen and thymus in the alcohol group was also observed. OSI was used as an indicative of alcohol’s toxic effects on these organs, resulting in a decreased proportion between the weight of the thymus and spleen and the body weight of animals exposed to alcohol during intrauterine development and lactation, confirming the reduction in size of these organs. This may result from the reduced number of splenic and thymic T cells, suppression of B cell proliferation, and delayed thymus development, which can be caused by the pro-oxidant action of ethanol (Zhang et al., 2012). However, it is worth noting that melatonin administration made the OSI of the thymus and spleen similar to that of the control group. This suggests that melatonin likely acts through its membrane receptors MT1, MT2, and nuclear receptors, which are present in the thymus and spleen, promoting the hormone’s effects on these organs and protecting against the harmful effects of alcohol on their morphophysiology (Carrillo-Vico et al., 2003; Bai et al., 2020). All these findings may explain the attenuation of damage in the thymus and spleen of 30-day-old offspring caused by ethanol during gestation and lactation in the alcohol + mel group.
5. CONCLUSION
In conclusion, the administration of melatonin simultaneously with alcohol consumption during gestation and lactation can be beneficial for the thymus and spleen of offspring from ethanol-consuming mothers. This hormone has shown high therapeutic and immunomodulatory potential in protecting and attenuating tissue, cellular, and molecular damage caused by excessive ethanol intake, thereby preventing elevated levels of apoptosis and pro-inflammatory cytokines in these organs of the immune system in the offspring.
REFERENCES
ABD-ALLAH, A. R. A.; EL-SAYED, M. E.; ABDEL-WAHAB, M. H.; HAMADA, F. M. A. Effect of melatonin on estrogen and progesterone receptors in relation to uterine contraction in rats. Pharmacol. Res., v. 47, p. 349 – 354, 2003.
ADAMS, C.; CONIGRAVE, J. H.; LEWOHL, J.; HABER, P.; MORLEY, K. C. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav Immun., v. 89, p. 501-512, 2020.
AHLUWALIA, B.; WESLEY, B.; ADEYIGA, O.; SMITH, D. M.; DA-SILVA, A.; RAJGURU, S. Alcohol modulates cytokine secretion and synthesis in human fetus: an in vivo and in vitro study. Alcoh. v. 21, n. 3, p. 207-13, 2000.
ALVES, C.M. et al. Morphometric analysis of the thymus of puppies infected with the Snyder Hill Strain of canine distemper virus. Arq. Bras. Med. Vet. Zootec., v.58, p.472-479, 2006.
ARAÚJO-FILHO, J. L. S.; MELO-JÚNIOR, M. R.; VEIGA, R. K. A.; MACHADO, M. C. F. P.; PATU, V. J. R. M.; PONTES-FILHO, N. T. Histomorphometric analysis of the hearts of exposed rats indirectly to ethanol and chronic malnutrition during perinatal period. R. Ci. méd. biol., v. 6, n. 1, p. 17-25, 2007.
BAI, J.; ZHANG, L.; ZHAO, Z.; LI, N.; WANG, B.; YANG, L. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunol. v. 160, n. 1, p. 52-63, 2020.
BAPTISTA, F. H.; ROCHA, K. B. B.; MARTINELLI, J. L.; AVÓ, L. R. S.; FERREIRA, R. A.; GERMANO, C. M. R.; MELO, D. G. Prevalence and factors associated with alcohol consumption during pregnancy. Rev. Bras. Maternal Health. Infant., v. 17, n. 2, 2017.
BARBIERI, R. L.; PARREIRA, S. F.; STUDART, S. V.; DA-SILVA, A. R.; DUARTE, I. S.; LEME, P. L. S. Stem cells hematopoietic niches and inflammatory response to different synthetic prosthesis implanted in rat with incisional hernias. ABCD. Arq. Bras. de Cirur. Digest. v.30, n. 2, p. 108–113, 2017.
BUDEC, M.; CIRIĆ, O.; KOKO, V.; AŜANIN, R. The possible mechanism of action of ethanol on rat thymus. Drug Alcohol Depend., v. 30, n. 2, p. 181-5, 1992.
BURD, L.; ROBERTS, D.; OLSON, M.; ODENDAAL, H. Ethanol and the placenta: A review. The Jour. of Matern.-fetal and Neonat. Med, v. 20, n. 5, p.361-375, 2007.
CARRILLO-VICO, A.; GARCÍA-PERGAÑEDA, A.; NAJI, L.; CALVO, J. R.; ROMERO, M. P.; GUERRERO, J. M. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system. Cell Mol Life Sci. v. 60, n. 10, p. 2272-8, 2003.
CHITIMUS, D. M.; POPESCU, M. R.; VOICULESCU, S. E.; PANAITESCU, A. M.; PAVEL, B.; ZAGREAN, L.; ZAGREAN, A. M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules. v. 10, n. 9, p. 1211, 2020.
COHEN-KEREM, R.; KOREN, G. Antioxidants and fetal protection against ethanol teratogenicity. I. Review of the experimental data and implications to humans. Neurotoxicol Teratol., v. 25, n. 1, 2003.
CORAZZA, G. R.; ADDOLORATO, G.; BIAGI, F.; CAPUTO, F.; CASTELLI, E.; STEFANINI, G. F.; GASBARRINI, G. Splenic function and alcohol addiction. Alcohol Clin. Exp. Res., v. 21, n. 2, p. 197-200, 1997.
COSTA, D. O.; NETO, P. F. V.; FERREIRA, L. N.; COQUEIRO, R. S.; CASOTTI, C. A. Consumption of alcohol and tobacco in pregnancy assisted by the family health strategy. Rev. Elet. Gestão & Saúde, v. 5, n. 3, p. 934-48, 2014.
CHEN, D.; ZHANG, F.; REN, H.; LUO, J.; WANG, S. Role of cytokines and chemokines in alcohol-induced tumor promotion. Onco Targets Ther. v.10, p.1665-1671, 2017.
DEAK, T.; KELLIHER, K. T.; WOJCIK, H. J.; GANO, A. Prenatal and adolescent alcohol exposure programs immunity across the lifespan: CNS-mediated regulation. Pharmacol Biochem Behav., v. 216, p. 173390, 2022.
EID, N.A.; ITO, Y.; LI, Z.; ABE, H.; KUSAKABE, K.; SHIBATA, M.A.; OTSUKI, Y. The relationship between apoptosis and splenocyte depletion in rats following ethanol treatment. Med. Electron microsc., v. 33, p. 89-95, 2000.
GHOSH, P.; DEY, T.; MAJUMDER, R.; DATTA, M.; CHATTOPADHYAY, A.; BANDYOPADHYAY, D. Insights into the antioxidative mechanisms of melatonin in ameliorating chromium-induced oxidative stress-mediated hepatic and renal tissue injuries in male Wistar rats. Food Chem Toxicol., v. 173, p. 113630, 2023.
GOLALIPOUR, M.J. et al. Morphometric alterations to the rat spleen following formaldehyde exposure. Folia Morphol., v.67, p.66-71, 2008.
GUPTA, K. K.; GUPTA, V. K; SHIRASAKA, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol Clin Exp Res., v.40, n. 8, p. 1594-602, 2016.
HAASTRUP, M. B.; POTTEGÅRD, A.; DAMKIER, P. Alcohol and breastfeeding. Basic Clin Pharmacol Toxicol., v. 114, n. 2, p. 168-73, 2014.
HOEK, J. B.; PASTORINO, J. G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. v. 27, n. 1, p. 63-68, 2002.
INCE, E.; CURABEYOĞLU, F.; AKYOL, S. Oxidative stress in lymphoid tissues and complement activation in alcoholic mother rats and their newborns. Gen Physiol Biophys., v. 38, n. 1, p. 91-100, 2019.
JOHNS, J. L.; CHRISTOPHER, M. M. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Veterin Pathol. v. 49, n. 3, p. 508-23, 2012.
JOSEPH, T. T.; SCHUCH, V.; HOSSACK, D. J.; CHAKRABORTY, R.; JOHNSON, E. L. Melatonin: the placental antioxidant and anti-inflammatory. Front Immunol., v. 15, p. 1339304, 2024.
KAPASI, A.A.; PATEL, G.; GOENKA, A.; NAHAR, N.; MODI, N.; BHASKARAN, M.; REDDY, K.; FRANKI, N.; PATEL, J.; SINGHAL, P.C. Ethanol promotes T cell apoptosis through the mitochondrial pathway. Immunology, v. 108, p. 313-320, 2003.
KELTY, E.; TERPLAN, M.; GREENLAND, M.; PREEN, D. Pharmacotherapies for the Treatment of Alcohol Use Disorders During Pregnancy: Time to Reconsider? Drugs., v. 81, p. 739–748, 2021.
KIM, C. H. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med., v. 1, p. 13-9, 2010.
KURHALUK, N. Alcohol and melatonin. Chronobiology International, v. 38, n. 6, p. 785–800, 2021.
LEE, E. S.; KIM, J. H.; IM, S.; LEE, K. B.; SOHN. S.; KANG, W. H. Application of computerized image analysis in pigmentary skin diseases. Internat. J. of Dermatology. v. 40, p. 45-49, 2001.
LIU, X.; CONNAGHAN, K. P.; WEI, Y.; YANG, Z.; LI, M. D.; CHANG, S. L. Involvement of the Hippocampus in Binge Ethanol-Induced Spleen Atrophy in Adolescent Rats. Alcohol Clin Exp Res., v. 40, n. 7, p.1489-500, 2016.
MARCO, E. M.; PEÑASCO, S.; HERNÁNDEZ, M. D.; Gil, A.; BORCEL, E.; MOYA, M.; GINÉ, E.; LÓPEZ-MORENO, J. A.; GUERRI, C.; LÓPEZ-GALLARDO, M.; RODRÍGUEZ DE FONSECA, F. Long-term effects of intermittent adolescent alcohol exposure in male and female rats. Front. Behav. Neurosci., v. 11, n. 233, p. 1-13, 2017.
MOLAD, M.; ASHKENAZI, L.; GOVER, A.; LAVIE-NEVO, K.; ZALTSBERG-BARAK, T.; SHAKED-MISHAN, P.; SOLOVEICHIK, M.; KESSEL, I.; ROTSCHILD, A.; ETZIONI, T. Melatonin Stability in Human Milk. Breastfeed Med., v. 14, n. 9, p. 680-682, 2019.
MOLINA, J. C.; GUERRERO-MORÁN, J. D.; GONZÁLEZ-ESPINOSA, C. Alcohol: Immunomodulatory Effects and Cancer. Rev Invest Clin., v. 75, n. 3, p. 129-142, 2023.
MORAES, C. L.; REICHENHEIM, M. E. Screening for alcohol use by pregnant women of public health care in Rio de Janeiro, Brazil. Rev Saude Pub., v. 41, n. 5, p. 695-703, 2007.
MOUSTAFA, A. M; EL-SAYED, E. M; BADARY, O. A; MANSOUR, A. M; HAMADA, F. M. A. Effect of bromocriptine on uterine contractility in near term pregnant rats. Pharmacol. Res., v. 39, n. 2, p. 89 – 95, 1999.
OBERHOLZER, M.; OSTREICHER, M.; CHRISTEN, H.; BRUHLMANN, M. Methods in quantitative image analysis. Histochemistry and Cell Biol. v. 105, p. 333-355, 1996.
PASCUAL, M.; MONTESINOS, J.; MARCOS, M.; TORRES, J. L.; COSTA-ALBA, P.; GARCÍA-GARCÍA, F.; LASO, F. J; GUERRI, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol., v. 22, n. 6, p. 1829-1841, 2016.
PRADIER, B.; ERXLEBE, E.; MARKERT, A.; RÁCZ, I. Microglial IL-1β progressively increases with duration of alcohol consumption. Naunyn Schmiedebergs Arch Pharmacol. v. 391, n. 4, p. 455-461, 2018.
REITER, R. J.; CALVO, J. R.; KARBOWNIK, M.; QI, W.; TAN, D. X. Melatonin and its relation to the immune system and inflammation. Ann N Y Acad Sci. v. 917, p. 376-86, 2000.
REITER, R. J.; MAYO, J. C.; TAN, D. X.; SAINZ, R. M.; ALATORRE-JIMENEZ, M.; QIN, L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res., v. 61, n. 3, p. 253-78, 2016.
REITER, R. J.; TAN, D. X.; QI, W.; MANCHESTER, L. C.; KARBOWNIK, M.; CALVO, J. R. Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept. v. 9, n. 3-4, p. 160-71, 2000.
RUMGAY, H.; MURPHY, N.; FERRARI, P.; SOERJOMATARAM, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients, v. 13, n. 9, p. 3173, 2021.
SANTOS, Y. B.; MELO, I. M. F.; ALVES, E. R.; NASCIMENTO, B. J.; SILVA, M. V.; FRANÇA, M. M. S.; PEREIRA, A. M.; SOARES, A. F.; TEIXEIRA, A. A. C.; TEIXEIRA, V. W. Melatonin and chronic ethanol consumption: effects on the offspring liver and kidney. Rev. Cient. Mult. Núcleo do Conhecimento, v. 04, p. 133-151, 2023.
SCHEIDT, L.; FRIES, G. R.; STERTZ, L.; CABRAL, J. C. C.; KAPCZINSKI, F.; ALMEIDA, R. M. M.; Ethanol during adolescence decreased the BDNF levels in the hippocampus in adult male Wistar rats but did not alter aggressive and anxiety-like behaviors. Trends Psychiat. Psychother., v. 7, n. 3, p. 143-151, 2015.
SEELIG, L. L. J.; STEVEN, W. M.; STEWART, G. L. Second generation effects of maternal ethanol consumption on immunity to Trichinella spiralis in female rats. Alcohol Alcohol. v. 34, n. 4, p. 520-528, 1999.
SILVA-GOTAY, A.; DAVIS, J.; TAVARES, E. R.; RICHARDSON, H. N. Alcohol drinking during early adolescence activates microglial cells and increases frontolimbic Interleukin-1 beta and Toll-like receptor 4 gene expression, with heightened sensitivity in male rats compared to females. Neuropharmacology. v. 197, p. 108698, 2021.
SLUKVIN, I.I.; JERRELLS, T. R. Different pathways of in vitro ethanol-induced apoptosis in thymocytes and splenic T and B lymphocytes. Immunopharmacology., v. 31, n. 1, p. 43-57, 1995.
STEINIGER, B. S. Human spleen microanatomy: why mice do not suffice. Immunology., v. 145, n. 3, p. 334-46, 2015.
VEIGA, R. K. A.; MELO-JÚNIOR, M. R.; ARAÚJO-FILHO, J. L. S.; MELLO, L. A.; PONTES-FILHO, N. T. Morphometric alterations in the thymus, spleen and peyer´s patches during pre and postnatal alcohol exposition. Rev. Elet. Farm., v. 4, n. 1, p. 32-42, 2007.
VARLINSKAYA, E. I.; SPEAR, L. P.; SPEAR, N. E. Acute effects of ethanol on behavior of adolescent rats: Role of social context. Alcohol Clin. Exp. Res., v. 25, n. 3, p. 377–385, 2001.
WANG, J. F.; SPITZER, J. J. Alcohol-induced thymocyte apoptosis is accompanied by impaired mitochondrial function. Alcohol., v. 14, n. 1, p. 99-105, 1997.
WEIBEL, E.R.; KISTLER, G.S.; SCHERLE, W.F. Practical stereological methods for morphometric cytology. J. Cell Biology, v.30, p.23-38, 1966.
WU, D.; CEDERBAUM, A. I. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health., v. 27, n. 4, p. 277-84, 2003.
WU, X.; CHENG, B.; CAI, Z. D.; LOU, L. M. Determination of the apoptotic index in osteosarcoma tissue and its relationship with patient’s prognosis. Can. Cell Inter., v.13, n. 56, p. 1-4, 2013.
ZHU, X.; SEELIG, L. L. J. Developmental aspects of intestinal intraepithelial and lamina propria lymphocytes in the rat following placental and lactational exposure to ethanol. Alcohol Alcohol. v. 35, n. 1, p. 25-30, 2000.
ZHANG, X.; LAN, N.; BACH, P.; NORDSTOKKE, D.; YU, W.; ELLIS, L.; MEADOWS, G. G.; WEINBERG, J. Prenatal alcohol exposure alters the course and severity of adjuvant-induced arthritis in female rats. Brain, Behav., and Immunity. v. 26, n. 3, p. 439-450, 2012.
[1] Master’s Degree in Animal Bioscience -UFRPE. ORCID: 0000-0002-6228-6951. Currículo Lattes: http://lattes.cnpq.br/1783975917572458.
[2] Master’s Degree in Animal Biosciences. ORCID: 0000-0002-4733-461X. Currículo Lattes: http://lattes.cnpq.br/1906334502843226.
[3] Master’s Degree in Animal Biosciences. ORCID: 0000-0001-9404-7501. Currículo Lattes: http://lattes.cnpq.br/8213260513385508.
[4] Master’s Degree in Animal Biosciences. ORCID: 0000-0002-5817-3743. Currículo Lattes: http://lattes.cnpq.br/4572948318160798.
[5] Graduated in Bachelor’s Degree in Biological Sciences. ORCID: 0000-0002-9662-0372. Currículo Lattes: http://lattes.cnpq.br/2810228771568915.
[6] Master in Gerontology – UFSM. ORCID: 0000-0001-9528-7312. Currículo Lattes: http://lattes.cnpq.br/9959642679541707
[7] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: https://orcid.org/0000-0003-1493-7964. Currículo Lattes: http://lattes.cnpq.br/9044747136928972.
[8] PhD in Morphology/UNIFESP-EPM; Master in Morphology/UNIFESPEPM. ORCID: 0000-0001-5940-9220. Currículo Lattes: http://lattes.cnpq.br/1539131079574469.
[9] Supervisor. PhD in Nuclear Technology from the University of São Paulo (USP), Master in Phytopathology from UFRPE. ORCID: 0000-0001-9533-5476. Currículo Lattes: http://lattes.cnpq.br/4292195468804301.
Material received: March 21, 2024.
Peer-Approved Material: July 29, 2024.
Edited material approved by the authors: September 3, 2024.







2 Responses
Agradeço por nos proporcionar conteúdos tão valiosos e bem elaborados.
Seu blog é uma fonte constante de inspiração. Obrigado!