ORIGINAL ARTICLE
LIMA, Iásly Costa [1], OLIVEIRA, Stephanie Lian Martins Kostk [2], LOPES, Roberta de Freitas [3], LOPES, Mariana Rodrigues de [4], ALVES, Pedro Gabriel Maia [5], CARVALHO, Isadora Oliveira de [6], CUNHA, Renildo Moura da [7], EVANGELISTA, Janaina Serra Azul Monteiro [8], ASSREUY, Ana Maria Sampaio [9]
LIMA, Iásly Costa et al. Barks of Dacryodes Kukachkana presents antiinflammatory effect in rat cutaneous wounds. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 09, Ed. 10, Vol. 01, pp. 188-205. October 2024. ISSN: 2448-0959, Acess link: https://www.nucleodoconhecimento.com.br/biology/dacryodes-kukachkana, DOI: 10.32749/nucleodoconhecimento.com.br/biology/dacryodes-kukachkana
ABSTRACT
Background: Dacryodes kukachkana (Burseraceae) is found in the Amazon rainforest of Brazil, being the genera widely used in folk medicine for several inflammatory conditions. Objective: In this study, it was investigated the anti-inflammatory effect of the Hydroethanolic Extract of D. kukachkana (HEDk) barks in the rat model of excisional skin wounds. Methods: The wounds were induced on the dorsal region of Wistar rats and treated daily by topical application of HEDk (500 mg/100 μL), compared to the control Saline (0.9% NaCl). Clinical parameters (edema, hyperemia, exudate, hypernociception), histopathological (leukocyte infiltrate, fibroblasts, blood vessels) and oxidative stress markers were evaluated from the 2nd to the 6th day post-ulceration. Results: HEDk reduced the following parameters at the 2nd day: inflammatory exudate (0 [0-0] vs. saline: 2 [1 – 3]); wound area (36%), healing index (48.56% ± 1.886 vs. saline: 27.99% ± 2.460) and malondialdehyde (27%). HEDk also reduced leukocyte infiltrate (49 – 34%) from the 4th to the 6th days post-ulceration. HEDk increased the parameters: nociceptive threshold (31 – 73%) from the 2nd to the 4th days; blood vessels (1.9x) and reduced glutathione (32%) at the 4th day; and the number of fibroblasts (91 – 44%) at the 2nd and 6th days. Conclusion: HEDk accelerates the healing process in the rat model of excisional cutaneous wounds via attenuation of inflammatory parameters and stimulation of fibroplasia and angiogenesis.
Keywords: Burseraceae, Breu-mescla, Inflammation, Plant polysaccharide, Wound healing.
1. INTRODUCTION
The wound healing is a physiological process that occurs after an injury. This process is divided into four overlapping phases: hemostasis, inflammation, proliferation and remodeling 1,2.
In the hemostatic phase, the body reacts to prevent and stop blood leakage. This phase is followed by the inflammatory phase, in which leukocytes migrate to the wound bed, increasing the production of Reactive Oxygen Species (ROS) to eliminate pathogenic microorganisms, along with deposition of new extracellular matrix. Once ROS production is exacerbated deleterious effects to the tissue formation may occur 3,4. Besides, classical clinical signs of inflammation are also present, such as: hyperemia, redness, edema, pain, tissue damage and function loss 5. The proliferative phase comprises epithelialization, angiogenesis, granulation tissue formation, fibroblast migration and collagen deposition. In the remodeling phase, collagen is shown more organized contributing to the reconstitution of the new extracellular matrix 3,6.
The nowadays healing therapy implies in diverse problems as low efficacy, invasiveness, patient discomfort and pain. In addition, the impaired wound healing may evolve to systemic inflammation, increasing the mortality rate associated to recurrent infections 7.
The genera Dacryodes belonging to the Burseraceae family is rich in volatile substances used worldwide in the folk medicine for the treatment of tonsillitis, diabetes, cancer8, headache, fever, malaria9, anemia, expectorant and skin diseases10. Experimental studies performed with the Dacryodes genus have demonstrated: hypoglycemic and antioxidant effects without apparent toxicity in rats for the methanol extract of Dacryodes edulis leaves11: antioxidant effect for D. rostrata fruits12; and antioxidant and antibacterial activities for the essential oil of the resins from D. buettneri13.
Dacryodes kukachkana L.O. Williams is a tall tree, popularly known in Brazil by “breu”, “breu-mescla” being widely found in the Amazonian Forest of Brazil and Peru14. For this species, an experimental study demonstrated the antinociceptive effect for the hydroethanolic extract obtained from its stem barks containing polyphenols, coumarins, flavonoids, saponins, taninns, terpenes and steroids15. Based on the results of Lopes and collaborators (2021), and the antinociceptive effect attributed to polyphenols, this study aimed to investigate the antiinflammatory effect of the hydroalcoholic extract obtained from D. kukachkana stem barks in the rat model of cutaneous wound.
2. MATERIAL AND METHODS
2.1 PLANT COLLECTION
Stem barks of Dacryodes kukachkana were collected in Mâncio Lima, Acre, Brazil from August to October 2018 (location 18 UTM 723548 7439155643 471), under authorization of Sisbio/ICMBio (n◦ 45912-1), being a voucher specimen deposited at the herbarium of the Federal University of Acre (n◦ 45912-1). The access activity was registered in the National System for the Management of the Genetic Heritage and the Associated Traditional Knowledge (SISGEN/MMA, code A76DD68), in accordance with the Brazilian Federal Law No. 13123/2015.
2.2 HYDROALCOHOLIC EXTRACT PREPARATION
Barks were dried (40 °C) during four days, grounded into fine particles and stored at room temperature (r. t.) until use. The plant material was subjected to percolation (72 h, r. t.) in 70% ethanol, filtered and concentrated in rotary evaporator (45 °C). The extract was frozen, lyophilized and named Hydroalcoholic Extract of D. kukachkana (HEDk)15.
2.3 ANIMALS
Female adult Wistar rats (8-10 weeks; 180 – 200 g) were maintained under controlled temperature (26 ± 1 °C) in light-dark cycle of 12 hours, receiving free access to food and water. The protocols were conducted in accordance to the National Institute of Health – NIH guidelines (publication nº 85-23, revised 2011) and approved by the Animal Care and Use Committee of the Federal University of Acre – Brazil (UFAC – N° 23107.018318/2017-18).
2.4 INDUCTION OF EXCISIONAL CUTANEOUS WOUNDS
Animals were weighed and subjected to dissociative anesthetic protocol with ketamine (100 mg/kg) and xylazine (10 mg/kg) by Intraperitoneal route (i.p.), followed by antisepsis with 2% chlorhexidine and dorsal trichotomy. Four excisional wounds were induced with surgical punch (7 mm diameter). The epidermis, dermis, and hypodermis were excised to expose the paniculus carnosus. The wounds were not sutured so that healing occurred by second intention16. In this model, it is possible to monitor healing by wound area, histological organization of connective tissue and aspects of re-epithelialization, and it was implemented in our research group based on the study developed by Pereira and collaborators (2016), as an ideal model for studying the physiological mechanisms involved in skin healing16.
2.5 EXPERIMENTAL GROUPS AND TREATMENT
The animals were divided into 2 experimental groups (n=6/group) and treated daily during 6 days by topical application of HEDk (500 mg/100 μL) or sterile saline (0.9%NaCl) as control. Euthanasia was performed at the post-surgical days 2, 4 and 6.
2.6 ASSESSMENT OF WOUND AREA AND WOUND HEALING INDEX
The wounds were photographed with a Sony DSC W110 digital camera, basic mode (no flash, no zoom, resolution 2 Mega Pixels) for measurement of wound area using ImageJ software. For image acquisition, it was used a stationary table on which the camera was fixed 20 cm from a ruler perpendicular to the ulcer. After obtention of the images and wound areas, the wound healing index was calculated using the formula: (initial area – final area) ÷ initial area × 100 17.
2.7 MACROSCOPIC EVALUATION OF INFLAMMATORY CLINICAL SIGNS IN THE CUTANEOUS WOUND
The inflammatory clinical signs edema, hyperemia and exudate were evaluated over the time course, on the 2nd, 4th and 6th days post-ulceration 17, by naked eye and measured semi-quantitatively, according to the intensity, by the scores: (0): absence; (1): mild; (2): moderate and (3): intense.
Edema was recorded by the swollen appearance in the bed and/or wound edges and confirmed, when necessary, by digital compression, observing the appearance of depression when it was present (Godet’s sign). Presence of hyperemia was recorded by the reddish or “flushed” appearance of the wound. When present, the exudate was scored for its intensity and appearance 17.
2.8 BEHAVIORAL ASSAY OF MECHANICAL HYPERNOCICEPTION
Hypernociception was measured by an electronic digital analgesimeter (Von Frey), coupled with a pressure transducer and a digital force (g) detector, adapted with a rigid disposable tip (0.5 mm2). The rats were individually placed in acrylic boxes, for a time adaptation period of 10 minutes and the hypernociception was evaluated by the application of a mechanical stimulus directly to the animal’s back, in the region immediately around the induced wound. Attempted escape from the stimulus, squealing or attacking the tip was considered as nociceptive response.
An experimental group of intact skin (without ulcer induction) was added to the evaluation of the healing inflammatory phase, aiming to measure the per se nociceptive response of the ulcer and compared to the treated groups. The value displayed on the force transducer was registered and three additional measurements were made. The average of the three measurements was considered a sample unit of one animal of each treatment (HEDK or 0.9% NaCl). This procedure was repeated for all animals at pre-established times: 0, 4, 6, 8, 12, 24, 48, 72 and 96 hours 17,18.
2.9 HISTOPATHOLOGICAL ANALYSIS
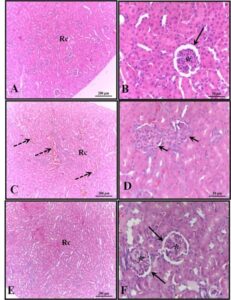
Skin samples immediately below the ulcer/reepithelization of each animal/ experimental group were fixed in 10% v/v formaldehyde and embedded in paraffin for preparation of hematoxylin and eosin stained slides (3 µm thickness). Samples (5 fields per slide/animal/experimental day) were photographed (40x magnification; Nikon Eclipse H550S microscope; Japan) and analyzed (Plugin “Cell Counter” ImageJ’s software – |NIH, EUA) for quantification of the following cells: inflammatory cells (neutrophils: cells with segmented and multilobular nucleus and mononuclear: cells with non-segmented nucleus and cytoplasm of varying amounts); fibroblasts cells (cells with eosinophilic cytoplasm and fusiform morphology, suggestive of fibroblasts/myofibroblasts differentiation); and well-differentiated blood vessels (excluding endothelial sprouts) 17.
2.10 MEASUREMENT OF OXIDATIVE STRESS MARKERS
Homogenates of skin samples were used to determine the tissue levels of the oxidative stress markers Malondialdehyde – MDA19 and Reduced Glutathione – GSH20, and the activity of Myeloperoxidase – MPO 21 by ELISA.
2.11 STATISTICAL ANALYSIS
Data was expressed as Mean ± S.E.M. and analyzed by Two-way ANOVA followed by Bonferoni test; scores of inflammatory clinical signs were expressed as Median (maximum and minimum) and analyzed by the Mann-Whitney’s test followed by the Dunn’s test. P<0.05 was considered significant.
3. RESULTS
3.1 HEDK REDUCES THE AREA OF WOUNDS AND INCREASES THE WOUND HEALING INDEX
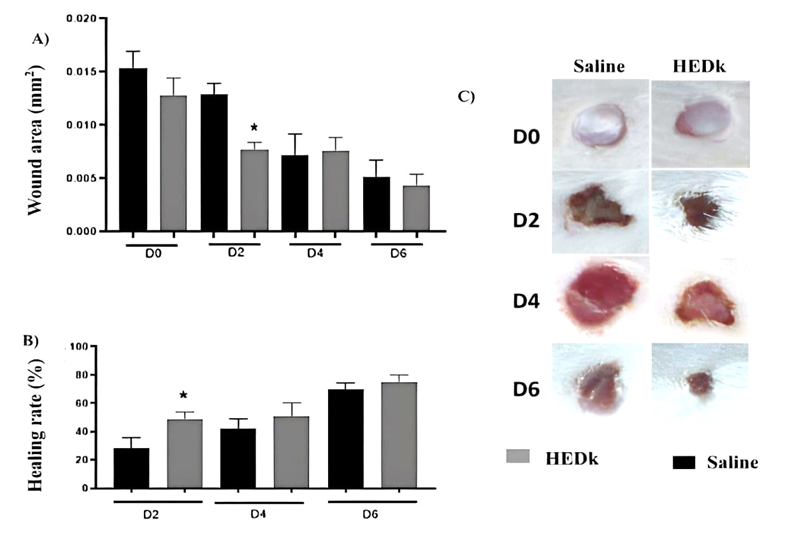
The wound area of non-treated animals, that had received saline (controls) showed a progressive decrease over the time course (2nd – 6th day). The treatment with HEDk reduced the wound area at the 2nd day post-ulceration (0.0076 ± 0.00026 vs. saline: 0.012 ± 0.00040 mm²) and the wound healing index increased at the 2nd day post-ulceration (48.56% ± 1.886 vs. saline: 27.99% ± 2.460) (Figure 1A-C).
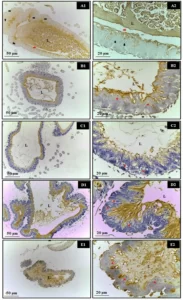
Figure 1. Effect of HEDk on the wound area and healing index over the healing time-course. Animals (n=6/group) were treated daily during 6 days by topical application of HEDk (500 mg/100 μL) or sterile saline (0.9%NaCl). Euthanasia was performed at the post-surgical days 2, 4 and 6. A) Wound area (Image J software); B) Wound healing index [(initial area – final area) ÷ initial area × 100]; C) macroscopic images. Mean ± S.E.M. (n=6). Two-way ANOVA/Bonferoni test.*p<0,05 vs. saline
3.2 HEDk REDUCES INFLAMMATORY CLINICAL SIGNS OF CUTANEOUS WOUNDS
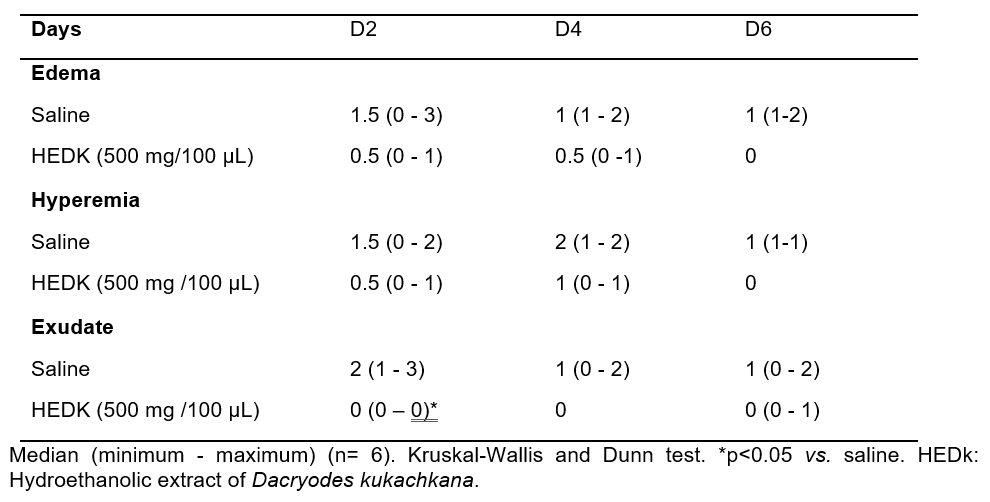
Control animals showed persistent edema, hyperemia and exudate over the healing time course. The treatment with HEDk reduced the exudate formation at the 2nd day post-ulceration, without significant alterations on the intensity of edema or hyperemia (Figure 1C, Table 1).
Table 1. Inflammatory clinical parameters evaluated macroscopically after treatment with HEDk
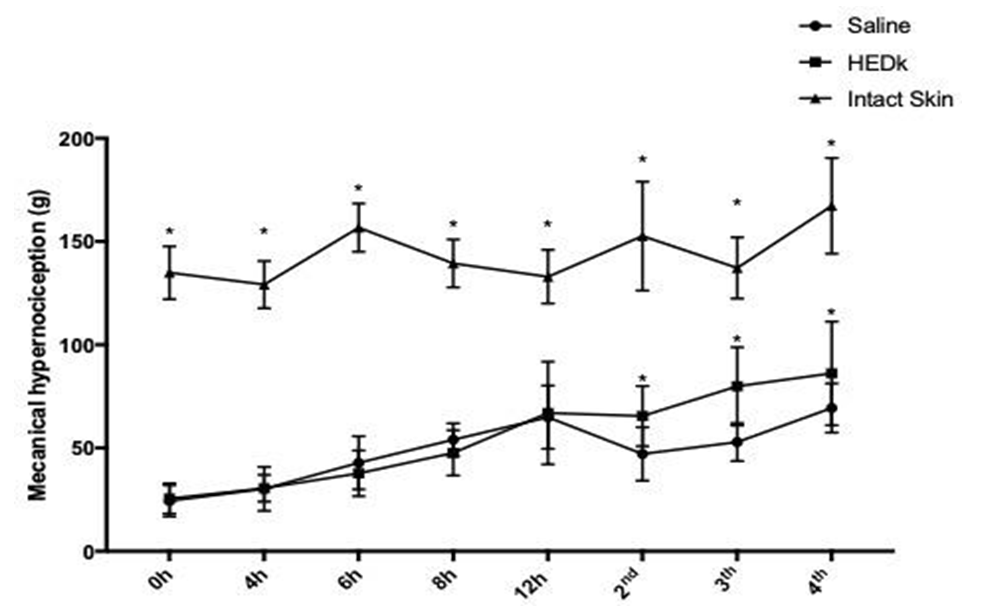
3.3 HEDK REDUCES THE HIPERNOCICEPTION OF CUTANEOUS WOUNDS
Intact skin animals showed regular and constant nociceptive response (2nd day: 142.23 ± 3.43 g; 3rd day: 137.79 ± 6.6 g; 4th day: 153.93 ± 4.82 g). The wounds of control animals showed reduced nociceptive response along the entire wound time- course (2nd day: 51.32 ± 1.08 g; 3rd day 49.84 ± 1.43 g; 4th day: 66.38 ± 2.31 g). The treatment with HEDk reduced the nociceptive response by 31% at the 2nd day, 73% at the 3rd and 60% at the 4th day post-ulceration (2nd day: 67.36 ± 2.85 g; 3rd day: 86. 50 ± 2.36 g; 4th day 106.62 ± 2.92) (Figure 2).
Figure 2. Effect of HEDk on the animal nociceptive response over the healing inflammatory phase. Animals (n=6/group) were treated daily during 6 days by topical application of HEDk (500 mg/100 μL) or sterile saline (0.9%NaCl). Hypernociception was evaluated (0-12 hours and at days 2- 4 post- ulceration) by the application of a mechanical stimulus directly to the wound edge. Mean ± S.E.M. (n=6) Two-way ANOVA/Bonferoni test. *p<0,05 vs. saline
3.4 HEDk ALTERS LEUKOCYTE INFILTRATE, FIBROBLASTS AND BLOOD VESSELS AT CUTANEOUS WOUNDS
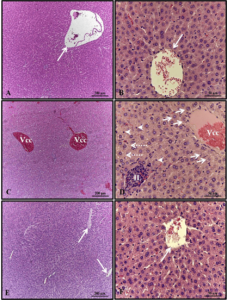
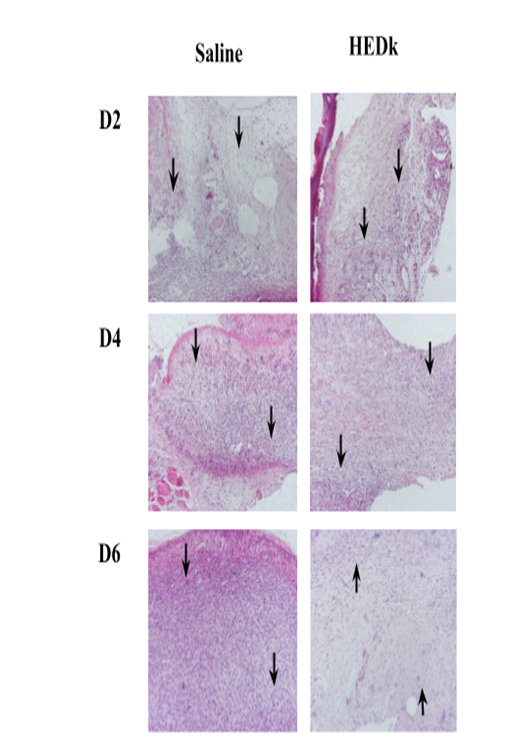
Quantitative analysis of histological slides showed that HEDk increased the number of fibroblasts by 91% (181.8 ± 10.48 vs. saline: 95.0 ± 10.65) at the 2nd day and by 44% (283.3 ± 19.54 vs. saline: 196.2 ± 5.72) at the 6th day. HEDk also increased the number of blood vessels in 1.9x (15.50 ± 5 vs. saline: 30.50 ± 2.39) at the 4th day but reduced the number of leukocytes (polymorphonuclear and mononuclear cells) by 51% (164.5 ± 16.98 vs. saline: 336.8 ± 16) at the 4th day and by 34% (215.4 ± 6.40 vs. saline: 329.3 ± 21.34) at the 6th day post-ulceration (Figure 3A-C; Figure 4).
Figure 3. Effect of HEDk on the number of fibroblasts and blood vessels. Animals were treated daily during 6 days by topical application of HEDk (500 mg/100 μL) or sterile saline (0.9%NaCl). Euthanasia was performed at days 2, 4 and 6 post-ulceration. (A) Fibroblasts; (B) blood vessels; (C) Leukocytes. Counts were performed in 5 fields/slide/H&E-stained. Mean ± S.E.M.(n=6). Two-way ANOVA/Bonferoni test. *p<0.05 vs. saline
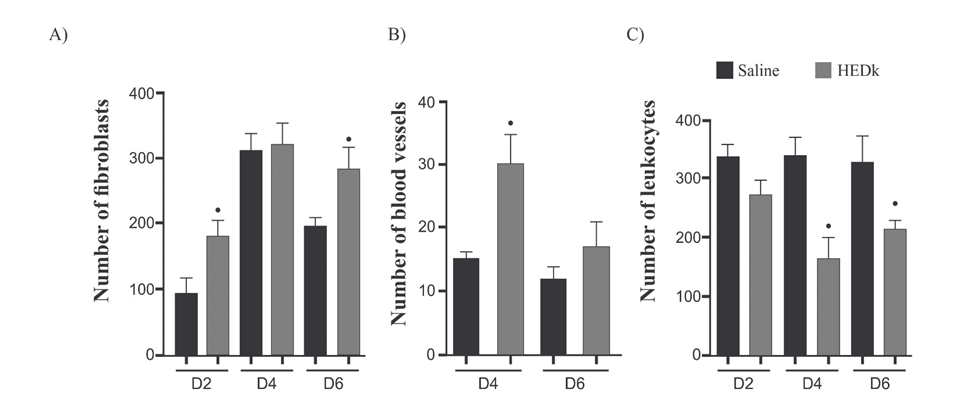
Figure 4. Effect of HEDk on the histological profile. Animals were treated daily during 6 days by topical application of HEDk (500 mg/100 μL) or sterile saline (0.9%NaCl). Euthanasia was performed at days 2, 4 and 6 post-ulceration. Slides H&E-stained of cutaneous wound at different time-points (Nikon Eclipse H550S microscope; 100x magnification). Two-way ANOVA/Bonferroni test. *p<0,05 vs. saline
3.5 HEDk ALTERS OXIDATIVE STRESS MARKERS
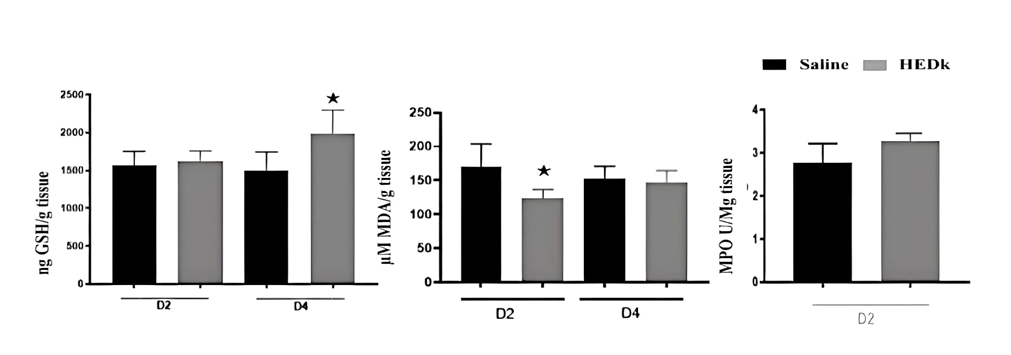
HEDk increased the concentration of GSH by 32% (1983 ± 128.3 vs. Saline: 1496 ± 124 ng/g tissue) at the 4th day post-ulceration and reduced that of MDA by 27% (123.5 ± 7.8 vs. saline: 171± 18.84 μM/g tissue) at the 2nd day post-ulceration. However, HEDk did not alter the activity of MPO at the 2nd day post-ulceration (2.832 ± 24.5 vs. Saline: 3.186 ± 30.5 U/g tissue) (Figure 5A-C).
Figure 5. Effect of HEDk on the oxidative stress markers. (A) Quantification of reduced glutathione (GSH), (B) malondialdehyde (MDA) and (C) myeloperoxidase activity (MPO) at tissue levels on cutaneous wounds. Two-way ANOVA/Bonferroni test and t-test. *p<0,05 vs. saline
4. DISCUSSION
Topical application of the Hydroethanolic Extract from D. kukachkana stem barks (HEDk) improved the healing process via increase of the healing rate, reduction of exudate, hypernociception, inflammatory cell infiltrate, and modulation of oxidative stress markers (GSH, MDA, MPO).
Hyperalgesia or inflammatory pain is also classified as a clinical sign observed in inflammatory processes, stimulating the sensitization of nociceptors by inflammatory mediators that are released from trauma, such as NO, PGs, TNF-α and IL-1β22,23. The painful response can be triggered by these mediators per se or they can act on the nociceptor, facilitating the transmission of the painful impulse24. The hyperalgesia evaluated by the application of mechanical stimulus in the ulcerated tissue leads to increased nociceptor sensitivity 16,25. In our study, the topical treatment with HEDk in cutaneous wounds reduced the hyperalgesia caused by mechanical stimulus from 48 to 96 hours. Accordingly, previous studies with HEDk demonstrated significant antinociceptive effect of HEDk in mice models of inflammatory pain induced by chemical (formalin/acetic acid) and thermal (hot plate) stimulus15.
In alignment with the described studies, the phytochemical prospection of HEDk by HPLC-UV-MS/MS revealed the presence of different polyphenols, such as phenolic acids (ellagic acid)26, tanins (castalagin derivate) and flavonoids (isorhamnetim-3-O-rhamnoside, ethyl gallate and epiafzelechin gallate), as major constituents 11,27. Besides, the antinociceptive activity had been already demonstrated for these molecules: isorhamnetin, a quercetin derivate28, ellagic acid29 and epiafzelechin gallate30. Thus, it is possible to be suggested, that the reduction of hyperalgesia caused by HEDk could be partially associated to the phenolic compounds present in the extract.
The inflammatory phase of the healing process is initiated with vascular and cellular events that occur simultaneously generating clinical signs inherent to the inflammatory phase (exsudate, hyperemia and edema) as a consequence of the change in vessel caliber favoring the outflow of leukocytes into the wound area31,32 . The treatment of cutaneous wounds with HEDk significantly reduced the exudate intensity, probably by the reduction of leukocytes infiltrate directly into the wound bed, favoring the healing process. In fact, HEDk reduced the number of leukocytes (polymorphonuclear and mononuclear cells) at the 4th day and the 6th day post-ulceration. These data are also in accordance with the demonstration of the anti-inflammatory activity for the HEDk main constituents (epiafzelechin gallate, isorhamnetin, quercetin derivate, ellagic acid) 28-30 .
Oxidative stress is a secondary physiological event associated to inflammation in which the persistence of dense inflammatory cells infiltrate cause cell damage4. Moreover, it is well known the antioxidant effect of polyphenols33. In our study, it was demonstrated that HEDk increases the concentration of GSH, and reduces that of MDA on wound tissues homogenate. As HEDk is composed by a mixture of phenolic compounds it is possible that the HEDk anti-inflammatory and antinociceptive effects are attributed to the anti-oxidant effect of its compounds.
The antinociceptive, anti-inflammatory and antioxidant effects of HEDk are consistent with the findings of other studies involving investigation of the healing process34, that correlates the reduced hyperalgesia and leukocyte infiltrate, that are responsible for the production of inflammatory and oxidative mediators involved at nociceptor sensitization35.
In the late phase of the healing process, fibroblasts are the main cells involved in the granulation tissue formation36. These cells produce elastin, fibronectin, glycosaminoglycans (extracellular matrix components) and proteases (responsible for debridement and physiological remodeling), and collagen (structural component of granulation tissue) responsible for tissue resistance and integrity, being essential for re-epithelialization2,37. HEDk increased the number of cells indicative of fibroblasts from the 2nd and 6th days post-ulceration. Complementarily, the formation of new blood vessels was stimulated at the 4th day post-ulceration in the cutaneous wounds after treated with HEDk. Thus, it is suggested that this extract acts in the proliferative phase of the healing process.
In summary, HEDk presents healing activity via reduction of the inflammatory phase, reducing the migration of inflammatory cells and hypernociceptive signals, modulating oxidative stress markers. HEDk also increased the proliferative phase stimulating fibroblast migration and new blood vessels formation.
The present study provides a novel phytotherapeutic strategy for the wound treatment based on bioactive compounds present in the Amazonian plant D. kukachkana.
5. CONCLUSION
The topical treatment of the Hydroethanolic Extract of Dacryodes kukachkana accelerates the healing process of excisional wounds in rats via modulation of the inflammatory and proliferative phases.
FUNDING
The authors thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (Funcap) for financial support.
CONFLICT OF INTEREST
The autors declare no conflict of interest.
ACKNOWLEDGMENTS
Conceptualization: A.M.S.A., I.C.L., S.L.M.K.O., R.F.L., R.M.D.; Data acquisition: I.C.L., I.O.C., M.R.L.; P.G.M.A., S.L.M.K.O.; Datal analysis: I.C.L., I.O.C. , S.L.M.K.O., M.R.L.; P.G.M.A., Extract isolation: R.M.C., R.F.L.; Writing—original draft preparation: I.C.L., A.M.S.A., M.R.L.; P.G.M.A., R.F.L., R.M.D, S.L.M.K.O.,. Writing—review and editing: A.M.S.A., I.C.L., I.O.C., M.R.L.; P.G.M.A., S.L.M.K.O. All authors have approved the final manuscript version.
REFERENCES
- Young A, Mcnaught CE. The physiology of wound healing and wound assessment. Surgery (Oxford). 2011; 29: 475-479. https://doi.org/10.1016/j.mpsur.2011.06.011.
- Wang PH, Huang BS, Horng HC, Yeh CC, Cheng YJ. Wound healing. Journal of the Chinese Medical Association. 2018; 81: 94 – 101. https://doi.org/10.1016/j. jcma.2017.11.002.
- Velnar T, Bailey T, Smrkolj V. The wound healing process: An overview of the cellular and molecular mechanisms. Journal of International Medical Research. 2009; 37: 1528-1542. https://doi.org/10.1177/147323000903700531.
- Coffman JA, Su YH. Redox regulation of development and regeneration. Current Opinion in Genetics & Development. 2019; 57: 9-15. https://doi.org/10.1016/j.gde.2019.06.002.
- Singh S, Young A, Mcnaught CE. The physiology of wound healing. Surgery (Oxford). 2017; 35 (9):473-477. https://doi.org/10.1016/ s0196-0644(88)80351-2.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiological reviews. 2019; 99 (1): 665-706. https://doi.org/10.1152/physrev.00067.2017.
- Pereira RF, Bartolo PJ. Traditional Therapies for Skin Wound Healing. Advances in Wound Care. 2016; 5: 208 – 229. https://doi.org/10.1089/wound.2013.0506.
- Omonhinmin CA. Ethnobotany of Dacryodes edulis (G Don) HJ Lam in Southern Nigeria 1: practices and applications among the Yoruba speaking people. Ethnobotany Research and Applications. 2012; 10: 175-184. https://doi.org/17348/era.10.0.175-184.
- Ndah NR et al. Ethnobotanical study of commonly used medicinal plants of the Takamanda Rainforest South West, Cameroon. African Journal of Plant Science. 2013; 7: 21-34. https://doi.org/10.5897/AJPS12.111.
- Ajibesin KK. Dacryodes edulis (G. Don) H. J. Lam: a review of its medical, phytochemicals and economical properties. Research Journal of Medicinal Plant. 2011; 5: 32-41. https://doi.org/10.3923/rjmp.2011.32.41.
- Ononamadu CJ et al. Toxicological study of aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis Toxicology Reports. 2020; 7: 909-918. https://doi.org/10.1016/j.toxrep.2020.07.007.
- Kong KW, Chew LY, Prasad KN, Lau CY, Ismail A, Sun J. Nutritional constituents and antioxidant properties of indigenous kembayau (Dacryodes rostrata (Blume) H. J. Lam) fruits. Food Research International. 2011; 44: 2332-2338. https://doi.org/10.1016/j.foodres.2010.10.039.
- Obame LC et al. Volatile components, antioxidant and antibacterial activities of Dacryodes buettneri J. Lam. essential oil from Gabon. Scientific Research and Essays. 2007; 2: 491-495. https://doi.org/10.5897/SRE.9000995.
- Daly DC. Notes on the Burseraceae in central Amazonia, including four new taxa. Studies in neotropical Burseraceae XXVI. Brittonia. 2018; 70: 427-444. https://doi.org/10.1007/s12228-018-9537-1.
- Lopes RF et al. Hydroethanolic extract from barks of the Amazonian species Dacryodes kukachkana: potential usage in painful conditions. European Academic Research. 2021; 9 (8): 5516-5528.
- Pereira LP et al. Modulator effect of a polysaccharide-rich extract from Caesalpinia ferrea stem barks in rat cutaneous wound healing: Role of TNF-α, IL-1β, NO, TGF-β. Journal of Ethnopharmacology. 2016; 187: 213-223. https://doi.org/10.1016/j.jep.2016.04.043.
- Lima IC et al. Galactomannan of Delonix regia seeds modulates cytokine expression and oxidative stress eliciting anti-inflammatory and healing effects in mice cutaneous wound. International Journal of Biological Macromolecules. 2022; 203: 342-349. https://doi.org/10.1016/j.ijbiomac.2022.01.144.
- Assreuy MAS et al. Polysaccharide-rich extract of Caesalpinia ferrea stem barks modulates inflammatory and proliferative phases enhancing diabetic cutaneous wound. Journal of Pharmacology and Toxicology. 2023; 18: 112-119. https://doi.org/10.1016/j.jep.2020.113501.
- Cighetti G, Debiasi S, Paroni R, Allevi P. Free and total malondialdehyde assessment in biological matrices by gas chromatography mass spectrometry: what is needed for an accurate detection. Analytical Biochemistry. 1999; 266: 222-229. https://doi.org/10.1006/abio.1998.2952.
- Sedlak J, Lindsay R. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Analytical Biochemistry. 1968; 25: 192-205. https://doi.org/10.1016/0003-2697(68)90092-4.
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of investigative dermatology. 1982; 78(3): 206-209. https://doi.org/10.1111/1523-1747.ep12506462″10.1111/1523-1747.ep12506462.
- Cunha TM, Verri JR, Silva WA, Poole JS, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proceedings of the National Academy of Sciences. 2005; 102: 1755-1760. https://doi.org/10.1073/pnas.0409225102.
- Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011; 25: 243-254. https://doi.org/10.1016/j.niox.2011.06.004.
- Malvar DC et al. Antinociceptive, anti-inflammatory and antipyretic effects of 1.5-diphenyl-1H-Pyrazole-3-carbohydrazide, a new heterocyclic pyrazole derivative. Life Sciences. 2014; 95: 81-88. https://doi.org/10.1016/j.lfs.2013.12.005.
- Möller KA, Johansson B, Berge OG. Assessing mechanical allodynia in the rat paw with a new electronic algometer. Journal of Neuroscience Methods. 1998; 84 (1): 41-47. https://doi.org/10.1016/S0165-0270(98)00083-1.
- De Souza MP et al. Phenolic and aroma compositions of pitomba fruit (Talisia esculenta Radlk.) assessed by LC–MS/MS and HS-SPME/GC–MS. Food Research International. 2016; 83: 87–94. https://doi.org/10.1016/j.foodres.2016.01.031.
- Atawodi SE et al. Evaluation of the Polyphenol Composition and Antioxidant Activity of African Variety of Dacryodes edulis (G. Don) H. J. Lam Fruit. Journal of Medicinal Food. 2009; 12: 1321-1325.https://doi.org/10.1089/jmf.2008.0215.
- Jamali-raeufy N, Baluchnejadmojar ADT, Roghani M, Keimasi S, Goudarz IM. Isorhamnetin exerts neuroprotective effects in STZ-induced diabetic rats via attenuation of oxidative stress, inflammation and apoptosis. Journal of Chemical Neuroanatomy. 2019; 102: 101709. https://doi.org/10.1016/j.jchemneu.2019.101709.
- Murphy MT et al. The polyphenol ellagic acid exerts anti-inflammatory actions via disruption of store-operated calcium entry (SOCE) pathway activators and coupling mediators. European Journal of Pharmacology. 2020; 15: 173036. https://doi.org/10.1016/j.ejphar.2020.173036.
- Min KR et al. (-)-Epiafzelechin: cyclooxygenase-1 inhibitor and antiinflammatory agent from aerial parts of Celastrus orbiculatus. Planta Med. 1999; 65: 460-462. https://doi.org/10.1055/s-2006-960813.
- Harper D, Young A, McNaught CE. The physiology of wound healing and wound assessment. Surgery (Oxford). 2011; 29: 475-479. https://doi.org/10.1016/j.mpsur.2011.06.011.
- Costa FLS, Tiussi LD, Nascimento MS, Côrrea ACS, Yasojima EY, Pires CAA. Diclofenac topical gel in excisional wounds maintain heal quality and reduce phlogistic signals. Acta Cirurgica Brasileira. 2014; 29(5): 328-333. https://doi.org/10.1590/S0102-86502014000500007.
- Shi L, Zhao W, Yang Z, Subbiah V, Suleria HAR. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environmental Science and Pollution Research. 2022; 29 (54): 81112-81129. https://doi.org/10.1007/s11356-022-23337-6.
- Adjafre BL et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. International Journal of Experimental Pathology. 2023; 105 (1): 33-44. https://doi.org/10.1111/iep.12498.
- Zhang JM, An J. Cytokines, Inflammation and Pain. International Anesthesiology Clinics. 2007; 45 (2): 27-37. https://doi.org/10.1097/aia.0b013e318034194e.
- Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cellular and Molecular Life Sciences. 2016; 73(20): 3861-85. https://doi.org/10.1007/s00018-016-2268-0.
- Singer AJ, Clark RAF. Cutaneous wound healing – Review. The New England Journal of Medicine. 1999; 341(10): 738-746. https://doi.org/10.1056/nejm199909023411006.
[1] Master’s degree in Physiological Sciences – UECE (stricto sensu); Bachelor’s degree in Physiotherapy – Unichristus. ORCID: https://orcid.org/0000-0002-7505-1801. Currículo Lattes: http://lattes.cnpq.br/3004418995568943.
[2] : Degree in Veterinary Medicine – UECE. ORCID: https://orcid.org/0009-0002-4000-7162. Currículo lattes: http://lattes.cnpq.br/9169592439040184.
[3] PhD in Biodiversity and Biotechnology of the Legal Amazon – BIONORTE – UFAC (stricto sensu); Master’s Degree in Science; Innovation and Technology for the Amazon – UFAC (stricto sensu); Bachelor’s Degree in Biological Sciences – UFAC. ORCID: https://orcid.org/0009-0002-4000-7162. Currículo Lattes: http://lattes.cnpq.br/5583276921036142.
[4] Degree in Biological Sciences – UECE. ORCID: https://orcid.org/0009-0007-3408-4432. Currículo Lattes: http://lattes.cnpq.br/6454517173628591.
[5] Bachelor’s Degree in Biomedicine – Estácio do Ceara. ORCID: https://orcid.org/0009-0004-8122-0570. Currículo Lattes: http://lattes.cnpq.br/5615160689434044.
[6] PhD in Physiological Sciences – UECE (stricto sensu); Master in Physiological Sciences – UECE (stricto sensu); Specialization in clinical hematology – Unileão (lato sensu); Degree in Biomedicine – Unileão. ORCID: https://orcid.org/0000-0002-9084-1991. Currículo Lattes: http://lattes.cnpq.br/0128591044401250.
[7] PhD in Bioactive Natural and Synthetic Products – UFPB (stricto sensu); Master in Animal Science – UFC (stricto sensu); Degree in Veterinary Medicine – FCAP/UPE. ORCID: https://orcid.org/0000-0003-2531-4631. Currículo Lattes: http://lattes.cnpq.br/3627410329724334.
[8] PhD in Pharmacology – UFC (stricto sensu), Master in Physiological Sciences – UECE (stricto sensu); Degree in Veterinary Medicine – UECE. ORCID: https://orcid.org/0000-0002-9583-1573. Currículo Lattes: http://Lattes.Cnpq.Br/2338988540733524.
[9] Supervisor. PhD in Pharmacology – UFC (stricto sensu); Master in Pharmacology – UFC (stricto sensu); Degree in Biological Sciences – UFC. ORCID: https://orcid.org/0000-0002-2323-5385. Currículo Lattes: http://lattes.cnpq.br/6315343480676626.
Material received: September 29, 2024.
Material approved by peers: October 4, 2024.
Edited material approved by authors: October 30, 2024.