ORIGINAL ARTICLE
FRANCO, Fernando Wendel [1], BOLIGON, Aline Augusti [2], MALONN, Maíra Casali [3], PUNTEL, Gustavo Orione [4], SOARES, Félix Alexandre Antunes [5]
FRANCO, Fernando Wendel. Et al. Phytochemical analysis of Artemisia absinthium aqueous extract. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 06, Ed. 02, Vol. 12, pp. 155-164. Frebuary 2021. ISSN:2448-0959, Access link in: https://www.nucleodoconhecimento.com.br/biology/phytochemical-analysis, DOI: 10.32749/nucleodoconhecimento.com.br/biology/phytochemical-analysis
ABSTRACT
Artemisia absinthium (AA) is a widely used medicinal plant in the world. Its aqueous extract is widely consumed in tea form for various health problems, especially gastric. This work aimed to investigate the phytochemical profile of AA aqueous extract (AAAE) as it is consumed by the population as a medicinal plant. The aqueous AA extract was prepared from dried leaves, and then lyophilized. In vitro analyses were performed on HPLC and phytochemical assays. Our results show that AAAE has several polyphenols and flavonoids, the main ones (ferulic acid, caffeic acid, and gallic acid) and (quercetin and kaempferol). These compounds have already described biological activities and may be responsible for the medicinal properties of the extract under study.
Keywords: polyphenols, flavonoids, absinthium, losna.
1. INTRODUCTION
For many years, there has been an increase in medicinal plant studies used for various therapeutic purposes (JARIĆ et al., 2007). It is believed that approximately 80% of the world population uses plants as the first therapeutic resource (ESTOMBA et al., 2006). In this context, Artemisia absinthium (AA) stands out because it is a medicinal plant with several biological activities and used by the population for many years (CHIASSON et al., 2001).
AA is an herbaceous plant with native fibrous roots from Central Europe, southern Siberia, North America, and Asia, where it is used as an herbal remedy (ABAD et al., 2012). Absinthium is the name originally given to the plant. In Brazil, for example, it is known as losna. It is believed to come from the Greek word “absinthium”, which means “unpalatable”, a reflection of its very bitter taste (BHAT et al., 2019).
Studies on the phytochemical profile of AA reveal the presence of several classes of chemical compounds, among which are: terpenoids, flavonoids, coumarins, polyphenols, sterols, and acetylenes. AA plant extracts have a broad spectrum of bioactivity, due to the presence of several active ingredients that work through various modes of action.
From an ethnopharmacological point of view, extracts from AA have traditionally been used and have several medicinal properties, such as hepatoprotective (WEI et al., 2019), an antidepressant (MAHMOUDI et al., 2009), diuretic (SINGH et al., 2012), anti-inflammatory (AHMAD, 1992), antifungal (SINGH et al., 2012), antimicrobial (EREL et al., 2012).
In Brazil, and many countries around the world, aqueous AA extract is consumed in the form of tea or simply as a cold macerated extract. This is why this work is important since it reproduces an extractive method similar to the empirical method used in folk medicine. Making it possible to know the phytochemical profile of tea in the main form that is consumed by the population.
2. MATERIAL AND METHODS
2.1 CHEMICAL REAGENTS
For high-performance liquid chromatography (HPLC-DAD) analysis de reagents methanol, ethanol, acetic acid, gallic acid, chlorogenic acid, ferulic acid, and caffeic acid were purchased from Merck (Darmstadt, Germany).
2.2 PREPARATION OF ARTEMISIA ABSINTHIUM AQUEOUS EXTRACT (AAAE)
AA leaves were collected from the herbarium of the Federal University of Santa Maria -RS, Brazil, in April of 2014. The botanical identification of the samples was confirmed and a voucher specimen (number ICN 9511; Artemisia absinthium) was deposited at the ICN Herbarium of the Federal University of Santa Maria (UFSM).
Aqueous AA extract (AAAE) was prepared from the dried leaves of the plant. First, the leaves were separated from the stems and then dried in the shade. Subsequently, they were weighed and placed in an aqueous extraction using a Soxhlet apparatus at 30 ° C.
The obtained solutions were then filtered and placed in volumetric flasks. They were then placed in a rotary evaporator at 30 ° C to remove the solvent and finally placed in a silica desiccator. Subsequently, the extract was lyophilized and the powder obtained stored at -80 ° C for further use.
2.3 QUANTIFICATION OF AAAE
2.3.1 HPLC ANALYSIS
HPLC-DAD of the AAAE was performed with a Shimadzu Prominence Auto Sampler (SIL-20A) HPLC system (Shimadzu, Kyoto, Japan), equipped with Shimadzu LC-20AT reciprocating pumps connected to a DGU 20A5 degasser with a CBM 20A integrator, SPD-M20A diode array detector, and LC solution 1.22 SP1 software.
Reverse phase chromatographic analyses were carried out under gradient conditions using a C18 column (4.6 mm x 250 mm) packed with 5 μm diameter particles; the mobile phase was water containing 2% acetic acid (A) and methanol (B), and the composition gradient was: 5% (B) for 2min; 25% (B) until 10 min; 40, 50, 60, 70 and 80% (B) every 10 min; following the method described by Boligon et al. (2012). The AAAE mobile phase was filtered through a 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath before use, the extracts were analyzed at a concentration of 20 mg/mL. The flow rate was 0.7 ml/min and the injection volume was 40μl. The sample and mobile phase was filtered through a 0.45 μm membrane filter (Millipore) and then degassed by ultrasonic bath before use. Stock solutions of standards references were prepared in the HPLC mobile phase at a concentration range of 0.030 – 0.250 mg/ml catechin, epicatechin, quercetin, quercitrin, isoquercitrin, kaempferol, and rutin, and 0.045 – 0.500 mg/ml for gallic, ferulic, chlorogenic and caffeic acids. Quantification was carried out by integration of the peaks using the external standard method, at 257 nm for gallic acid, 280 nm for catechin and epicatechin, 325 nm for chlorogenic, ferulic, and caffeic acids, and 365 for quercetin, quercitrin, isoquercitrin, rutin, and kaempferol. The chromatography peaks were confirmed by comparing its retention time with those of reference standards and by DAD spectra (200 to 600 nm). All chromatography operations were carried out at an ambient temperature and in triplicate.
2.3.2 DETERMINATION OF TOTAL PHENOLS
Total phenols were determined colorimetrically as described by (FOLIN-CIOCALTEU, 1973) with some modifications. Sample (1g) was mixed with 10 mL 80% methanol in a dark bottle and shaking for 2h. The color was developed by Folin-Ciocalteu reagent and sodium carbonate. A volume of 0.250 mL was mixed with 0.250 mL Folin-Ciocalteau reagent and 0.50 mL of 10% sodium carbonate (Na2CO3) and the volume was completed to 5 ml with distilled water. After incubation in dark at room temperature for 30 min, the absorbance of the reaction mixture was measured at 725 nm against a blank. Gallic acid was used as a standard compound and results were expressed in mg/mL.
2.3.3 DETERMINATION OF TOTAL FLAVONOIDS
Total flavonoids were determined according to the methods of the sample (1g) was mixed with 10 mL 80% methanol and shaking for 2h. Total flavonoids extract (0.4 mL) was added to 4 mL of H2O. Then 0.3 mL of 5% NaNO2 was added. After 5 min, 0.3 mL of 10% AlCl3 was added. After 6 min, 2 mL of 1 M NaOH was added and the total volume was made up to 10 mL with distilled water. The color was measured at 510 nm against a blank reagent. Catechin was served as a standard compound and results were expressed in mg/mL.
3. RESULTS
3.1 QUANTIFICATION OF AAAE CONSTITUENTS
3.1.1 HPLC-DAD
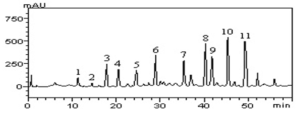
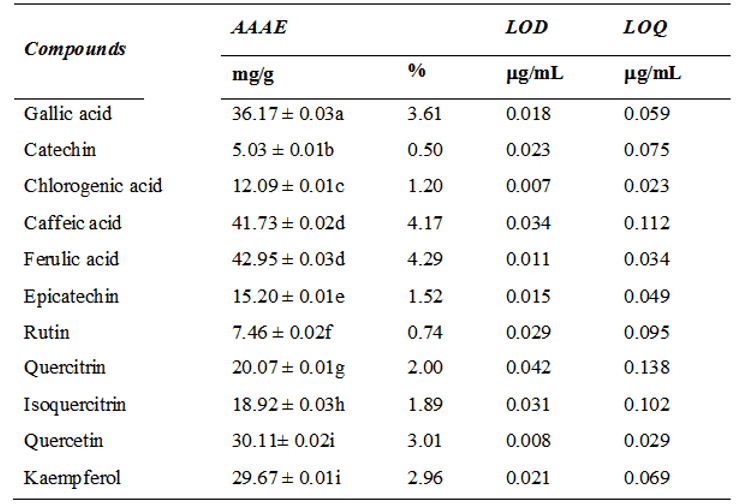
The HPLC profile of AAAE is shown in Figure 1 and Table 1 reveled the following main compounds: gallic acid (retention time-tR 11.39 min, peak 1), catechin (tR = 14.97 min, peak 2), chlorogenic acid (tR = 18.73 min, peak 3), caffeic acid (tR = 21.08 min, peak 4), ferulic acid (tR = 24.11 min, peak 5); epicatechin (tR = 29.37 min, peak 6), rutin (tR = 35.16 min, peak 7), quercitrin (tR = 39.85 min, peak 8), isoquercitrin (tR = 42.07 min, peak 9), quercetin (tR = 44.81 min, peak 10) and kaempferol (tR = 49.73 min, peak 11).
Figure 1: High-performance liquid chromatography phenolics and flavonoids profile of Artemisia absinthium aqueous extract (AAAE). Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), ferulic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), isoquercitrin (peak 9), quercetin (peak 10) and kaempferol (peak 11).
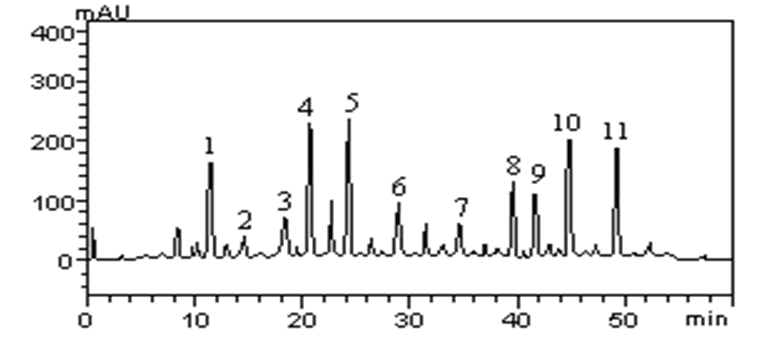
Table 1 – HPLC phenolics and flavonoids composition of Artemisia absinthium aqueous extract (AAAE). Results are expressed as mean ± S.E. of three determinations. LOD limit of detection (µg/ml), LOQ limit of quantification (µg/ml). Averages followed by different letters differ by the Tukey test at p < 0.05.
3.1.2 TOTAL PHENOLS AND FLAVONOIDS CONTENT
The major concentrated polyphenols founded were ferulic acid followed by caffeic acid and gallic acid. In flavonoid composition, the major concentration in AAAE was quercetin followed by kaempferol. These and other phenolic and flavonoid compounds founded and their concentrations were shown in Table 2.
Table 2 – Phytochemical analysis of total phenols and flavonoids content Artemisia absinthium aqueous extract (AAAE). Results are expressed as (mg of GAE/g of extract) and (mg of Quercetin/g of extract).
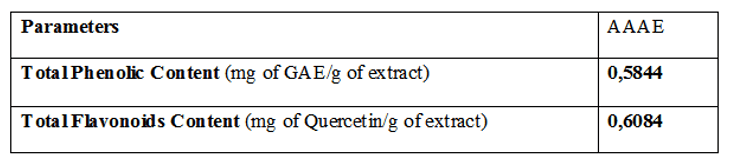
4. DISCUSSION
Artemisia absinthium is a plant species rich in polyphenols and flavonoids (MSAADA et al., 2015). Many of the biological activities of AA are described in the literature about these compounds found in aqueous extracts such as caffeic acid, ferulic acid, gallic acid, quercetin, and kaempferol. According to (ARAÚJO et al., 2019) caffeic acid and its derivatives have antimicrobial activity against Escherichia coli and Staphylococcus aureus. Pernin et al., (2019) demonstrated that ferulic acid has antimicrobial activity against Listeria monocytogenes. Also, according to Borges et al., (2013) gallic acid has antimicrobial effects on Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Listeria monocytogenes. In another study, Pernin et al., (2019) show that ferulic acid has antimicrobial activities in front of Listeria monocytogenes.
A. absinthium also contains flavonoids such as quercetin, rutin, and other flavonoids like such as isoquercitrin, quercitin-3-O-d-glucoside (OLENNIKOV et al., 2018), quercitin-3-Orhamnoglucoside (PERNIN et al., 2019), isorhamnetin-3-O-rhamnoglucoside, isorhamnetin-3-glucoside, and phenolic acids such as chlorogenic, syringic, coumaric, salicylic, and vanillic that are probably involved in the mechanism of prevention of oxidative damages in biological systems (KORDALI et al. 2005b).
Several studies have shown that A. absinthium possesses potent antioxidant, free radical scavenging, and anti-inflammatory activity. AA has a high content of phytochemicals such as total phenolic compounds and total flavonoids, suggesting that these compounds contribute to the antioxidative activity. Phenolic substances such as flavonols, cinnamic acids, coumarins, and caffeic acids or chlorogenic acids are believed to have antioxidant properties, which may play an important role in protecting cells and any organ from oxidative degeneration (WISEMAN et al., 2001).
All the biological properties described in the individual or mixed components, as they occur in the aqueous extract, may be responsible for the medicinal properties attributed to the aqueous extract. Thus, the phytochemical analysis of AA performed in this work contributes to try to elucidate or explain one of the possible mechanisms of action that corroborate the therapeutic use of AA in folk medicine.
5. CONCLUSION
From this study, we can conclude that AAAE has high polyphenolic and flavonoids contents. In general, we can see that these compounds have many biological activities, like antifungal, antimicrobial, and antioxidant. The various biological properties of AA extract, consumed by the population for medicinal purposes, maybe due to the additive or synergistic effect of the various components present in the plant. Since these components present in the aqueous extract, by themselves, already have biological activity described in the scientific literature. However, further studies are needed to highlight the properties of AAAE.
ACKNOWLEDGMENT
Financial support by the Rio Grande do Sul Research Support Foundation (FAPERGS). FWF received a fellowship from the FAPERGS and was involved in all the steps of this paper construction.
REFERENCES
ABAD, María José et al. The Artemisia L. genus: a review of bioactive essential oils. Molecules, v. 17, n. 3, p. 2542-2566, 2012. https://doi.org/10.3390/molecules17032542
AHMAD, F.; KHAN, R. A.; RASHEED, S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scariola and Artemisia absinthium. Journal of Islamic Academy of Sciences, v. 5, n. 2, p. 111-114, 1992.
ARAÚJO, Marianna O. et al. Synthesis, antibacterial evaluation, and QSAR of caffeic acid derivatives. Journal of Chemistry, v. 2019, 2019. https://doi.org/10.1155/2019/3408315
BHAT, Rahil Razzak et al. Chemical composition and biological uses of Artemisia absinthium (wormwood). Plant and Human Health, v. 3, p. 37-63., 2019. https://doi.org/10.1007/978-3-030-04408-4_3
BORGES, A., FERREIRA, C., SAAVEDRA, M.J., SIMÕES, M., 2013. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microbial Drug Resistance v. 19, p. 256–265., 2013. https://doi.org/10.1089/mdr.2012.0244
CHIASSON, H., BÉLANGER, A., BOSTANIAN, N., VINCENT, C., POLIQUIN, A. (2001). Acaricidal properties of Artemisia absinthium and Tanacetum vulgare (Asteraceae) essential oils obtained by three methods of extraction. Journal of economic entomology, v. 94(1), p. 167-171.,2001. https://doi.org/10.1603/0022-0493-94.1.167
EREL, Ş. B., Reznicek, G., Şenol, S. G., YAVAŞOĞLU, N. Ü. K., Konyalioğlu, S., & Zeybek, A. U. (2012). Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turkish Journal of Biology, v. 36(1), p. 75-84., 2012.
ESTOMBA, D., LADIO, A., LOZADA, M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. Journal of Ethnopharmacology, v. 103, n. 1, p. 109-119, 2006. https://doi.org/10.1016/j.jep.2005.07.015
JARIĆ, Snežana et al. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia). Journal of ethnopharmacology, v. 111, n. 1, p. 160-175, 2007. https://doi.org/10.1016/j.jep.2006.11.007
KORDALI, Saban et al. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. Journal of agricultural and food chemistry, v. 53, n. 5, p. 1408-1416, 2005. https://doi.org/10.1021/jf048429n
MAHMOUDI, M., EBRAHIMZADEH, M. A., ANSAROUDI, F., NABAVI, S. F., NABAVI, S. M. (2009). Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. African journal of Biotechnology, v. 8(24)., 2009. DOI: 10.4314/ajb.v8i24.68818
MSAADA, K., SALEM, N., BACHROUCH, O., BOUSSELMI, S., TAMMAR, S., ALFAIFY, A., AL SANE, K., BEN AMMAR, W., AZIZ, S., HAJ BRAHIM, A., HAMMAMI, M., SELMI, S., LIMAM, F., MARZOUK, B., 2015. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood ( Artemisia absinthium L.) Essential Oils and Phenolics. Journal of Chemistry. p. 1–12., 2015 https://doi.org/10.1155/2015/804658
OLENNIKOV, Daniil N. et al. Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Frontiers in pharmacology, v. 9, p. 756, 2018. https://doi.org/10.3389/fphar.2018.00756
PERNIN, A., BOSC, V., MAILLARD, M.N., DUBOIS-BRISSONNET, F., 2019. Ferulic acid and eugenol have different abilities to maintain their inhibitory activity against Listeria monocytogenes in emulsified systems. Frontiers in Microbiology v. 10, p. 1–10., 2019. https://doi.org/10.3389/fmicb.2019.00137
SINGH, Rajeev; VERMA, Pawan Kumar; SINGH, Gagandeep. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. Journal of Complementary Medicine Research, v. 1, n. 2, p. 101-104, 2012. https://doi.org/10.5455/jice.20120525014326
WEI, Xianxian et al. The Extracts of Artemisia absinthium L. suppress the growth of hepatocellular carcinoma cells through induction of apoptosis via endoplasmic reticulum stress and mitochondrial-dependent pathway. Molecules, v. 24, n. 5, p. 913, 2019. https://doi.org/10.3390/molecules24050913
WISEMAN, Sheila; MULDER, Theo; RIETVELD, Anton. Tea flavonoids: bioavailability in vivo and effects on cell signaling pathways in vitro. Antioxidants and Redox Signaling, v. 3, n. 6, p. 1009-1021, 2001. https://doi.org/10.1089/152308601317203549
[1] Master in Toxicological Biochemistry, PhD student in Pharmacology.
[2] Doctorate in Pharmaceutical Sciences.
[3] Master in Analytical Chemistry.
[4] Advisor. Doctorate in Toxicological Biochemistry.
[5] Doctorate in Toxicological Biochemistry.
Submitted: December, 2020.
Approved: Frebuary, 2021.