ORIGINAL ARTICLE
MOREIRA, Luciano Gonçalves [1], MARTINS, João Victor Oliveira [2], ROSA, Jonathan Trovato Martins [3], DUARTE, Larissa Beatriz Cantarino [4], NASCIMENTO, Elaine Belchior do [5]
MOREIRA, Luciano Gonçalves. et al. Feasibility study of the use of an electromechanical system for automating a manual resuscitator in patients with breathing difficulties. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year. 07, Ed. 05, Vol. 01, pp. 203-233. May 2022. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/technology-en/breathing-difficulties
ABSTRACT
Due to the COVID-19 pandemic, experienced since March 2020, quick-to-apply coping actions are necessary. As widely reported in the press, artificial respirators are very important equipment in the fight against COVID-19, especially in severe cases. Due to its high cost, research has been carried out aiming at the development of low-cost artificial respirators, through the automation of the manual resuscitator known as AMBU. In this context, researchers from the Santos Dumont campus of the Instituto Federal do Sudeste de Minas Gerais developed a similar prototype. That said, aiming to answer the following guiding question: is the development of low-cost artificial respirators based on the automation of manual resuscitators feasible for the care of people with respiratory impairment? This work aims to evaluate the viability of the prototype developed on that campus, in emergency care for patients with breathing difficulties. For this, pre-clinical tests were carried out on benches, with a test lung and an inflatable CPR dummy, among other equipment capable of validating its operation. In this context, its operation was demonstrated and the results of the tests were submitted to the evaluation of a panel composed of specialists in the health area, in order to prove, through forms and clinical reports, if the prototype is viable for what it proposes and if is able to be produced by a company authorized by ANVISA and tested on humans, in addition to indicating in which situations it can be used, as directed by ANVISA’s RDC 386/2020. In this way, the panel composed of six evaluators evaluated the performance of the prototype in five categories: Usability, Safety, Electrical Safety, Mechanical Ventilation and Feasibility. For this, they analyzed 54 propositions related to the mentioned categories, thus totaling 324 evaluations, of which, according to the evaluators, 90% of these attested that the prototype is easy to use, safe, provides adequate ventilation and is feasible care for patients with different levels of breathing difficulties. Therefore, after the evaluations, it was found that the project is able to be produced by a company authorized by ANVISA, and can be tested on humans, observing the criteria of ANVISA’s RDC 386/2020.
Keywords: COVID-19, Manual Resuscitator, Automation, Low cost, Arduino.
1. INTRODUCTION
As widely reported in the world press, since March 2020, the world has been living under the COVID-19 pandemic and, since then, several countries have sought solutions to mitigate its impacts. COVID-19 is a disease caused by the Sars-Cov-2 virus, in which about 80% of infected people recover from the disease without needing hospital treatment, however, one in six infected people becomes seriously ill and develops difficulties with breathe (ORGANIZAÇÃO PAN-AMERICANA DE SAÚDE, 2020). In this context, it is estimated that approximately 5% of patients with COVID-19 suffer from the so-called Acute Respiratory Distress Syndrome (ARDS) and require assisted breathing, thus artificial respirators are the last hope for most severely affected patients. At first, 5% may seem low, but, in the case of a highly contagious disease, the number of patients who need this equipment can increase very quickly and put health systems around the world at risk (WALLACE, 2020).
Artificial respirators or mechanical ventilators are, according to the company Vitalaire (2021, p. 1),
suporte oferecido, por meio de um aparelho, ao paciente que não consegue respirar espontaneamente por vias normais devido a fatores como doenças, anestesia e anomalias congênitas. Os ventiladores também são usados para permitir descanso dos músculos respiratórios até que o paciente seja capaz de reassumir a ventilação espontânea.
According to data from the Ministry of Health apud Vidale (2020), “Brazil currently has 65,411 respirators, of which 46,663 are available in the Health Unic System (SUS)[6] and 18,748 in the private network. However, only 61,219 are fit for use. In a normal period, the amount would be sufficient, but in the current scenario, probably not”, that is, the number of sufficient respirators is reduced taking into account the pandemic, and the fear of the lack of this equipment in serving the population is great. In urgent and emergency care, where artificial respirators are not available, health professionals use manual resuscitators to provide initial ventilatory support, until the patient returns to breathing on their own or receives adequate care through a respirator. In this context, researchers all over the world have developed low-cost mechanical respirators and/or ventilators that can quickly serve the population, many of them based on the automation of manual resuscitators. Likewise, researchers from the Santos Dumont campus, at the Instituto Federal do Sudeste de Minas Gerais, during an extension project to combat COVID-19, developed the prototype of an Electromechanical System (ES) system for automatically pressing the bag of the manual resuscitator.
Therefore, in the case of the second phase of the Santos Dumont campus extension project, this research sought to answer the following guiding question: is the development of low-cost artificial respirators based on the automation of manual resuscitators feasible for the care of people with respiratory impairment? Thus, to answer this question, the general objective was to evaluate the viability of the prototype developed on the aforementioned campus, in emergency care for patients with breathing difficulties. For this purpose, pre-clinical bench tests were carried out, under the supervision of a panel composed of health professionals, in order to evaluate the previously mentioned prototype, developed by researchers from the aforementioned campus, and to prove whether it is viable for the care for patients with breathing difficulties.
To carry out this study, it was necessary to: carry out the tests and evaluations based on the guidelines of ANVISA’s RDC 386 / 2020; use materials and equipment that demonstrate their application through tests on benches, such as a test lung, CPR dummy, among others; select at least 5 different health professionals to compose the panel of judges, supervise the tests and evaluate the results; apply a questionnaire to the members of the panel of judges, in order to identify whether the prototype is viable for the care of patients with breathing difficulties; and ask the members of the panel of judges to issue clinical reports indicating the use of the prototype.
That said, the work is divided as follows: Section 2 presents a bibliographical review of the main themes related to the work, Section 3 presents the study methodology and tests, Section 4 presents the results and discussions and Section 5 presents the conclusion.
2. LITERATURE REVIEW
For emergency situations in which the patient cannot breathe on their own, as artificial respirators are not immediately available, health professionals use the Manual Resuscitator (MR) or Bag-Valve-Mask (BVM) device, popularly known as AMBU, to provide ventilatory support to the patient until he starts breathing on his own again or receives hospital care with advanced resources. Therefore, these are indispensable in some places, such as: ICU, emergency rooms, ambulances and Fire Department (DIMAVE, 2019).
In the context of the pandemic, described in the previous section, an example of the emergency use of the BVM in this situation of lack of beds with respirators, was addressed in the BBC NEWS report (2021, p. 1), under the title: “Covid em Manaus: sem oxigênio, pacientes dependem de ventilação manual para sobreviver em Manaus“, where it is appropriate to highlight the following excerpts from the report by physician Pierre Souza:
A ventilação manual tem sido frequente, e aconteceu desde os primeiros meses de pandemia. […] Essa situação caótica muitas vezes exigiu passar noites inteiras ao lado do leito do paciente, fazendo uma escala do ambu, com colegas, técnicos e enfermeiros se revezando por longos períodos — às vezes meia hora, uma hora, uma hora e meia.
Thus, researchers around the world have developed low-cost mechanical respirators and/or ventilators that can quickly serve the population, many of them based on BVM automation. Even before the pandemic, Al Husseini et al. (2010), at MIT, were already developing a low-cost respirator. Their work describes the stages of development, testing and evaluation of different mechanisms for pressing the BVM bag on the device, which also has sensors that monitor ventilatory support for the patient and an Arduino platform to control the operation. Only bench tests were carried out at work.
Currently, the project’s official website at MIT[7] already contains preclinical and clinical tests on animals and humans, and as a result, its project served as an inspiration for the development of low-cost mechanical ventilators worldwide. Similarly, Shahid (2019) also developed a low-cost respirator, with the aim of further reducing costs compared to MIT, testing and evaluating different mechanisms for pressing the BVM bag and patient breathing sensors; for control, it also used the Arduino platform, however, it did not carry out pre-clinical or clinical tests, only bench tests. Both works evaluated that the use of a cam mechanism to press the BVM bag is the best option. Following the trend, several researchers around the world have developed similar prototypes of artificial respirators in the context of the pandemic, such as the AMBU Spur II developed by Domènech-Mestres et al. (2021), the MADVent developed by Vasan (2020), the RC Device developed by Chauhan et al. (2021), among others.
It is no different in Brazil, several public and private institutions have been working on the development of less complex technologies, such as, for example, the one created by the Polytechnic School of USP, in which Lopes (2020, p. 1) stated that:
criou um ventilador pulmonar emergencial de baixo custo que poderá atender a pacientes do coronavírus. O protótipo, chamado Inspire, pode ser montado em duas horas e não exige peças importadas. Anunciada a sua criação na semana do dia 27 de março, o respirador custa apenas mil reais, enquanto aqueles disponíveis atualmente custam, em média, quinze mil reais.
In addition to this, Lopes (2020) also mentions the Instituto Mauá de Tecnologia which, in partnership with Mercedes-Benz, developed “an adaptation of the Ambu, a mechanical ventilator commonly used by doctors and rescuers. The functioning of the new respirator, which will be produced in São Bernardo, will depend on an engine like the one used in windshield wipers”. That said, several other initiatives are under development in the country, such as VENT 19 developed by Tsuzuki et al. (2020), which also uses a 12 v, 30 W brush motor and cam mechanism for pressing the BVM bag, which only performed tests on benches, with the next phase of pre-clinical tests with a test lung and in animals yet to be performed.
In common, all these projects aim to obtain a similar operation to the artificial respirators available on the market, which must, in addition to providing ventilatory support, monitor the patient’s condition during ventilation. For this very reason, there are many challenges in the development of respirators based on BVM automation. Well, basically, according to the instruction manuals of some of the main BVM models available on the market, such as those of the manufacturers: Protec Export (IWASA, 2021), Cirúrgica Fernandes (HIGA, 2021), Domax (CUNHA, 2021), RWR (POTONYACZ, 2021) and Besmed (MORIYA, 2021), the function of a BVM is to generate airflow to the patient by pressing the self-inflating bag. Therefore, to identify the correct ventilation, the health professional must observe the expansion of the patient’s chest when the bag is pressed, as well as listening for an expiratory flow through the patient’s valve and observing the chest descent when releasing the handbag. During this process, it is important that the professional is also aware of signs of cyanosis, such as the color of the face or eyes; fogging of the patient’s mask; insufficient ventilation; airway pressure; formal function of all valves; function of the reservoir and oxygen tubing. This procedure must be repeated until the patient is able to breathe on his own again (IWASA, 2021; HIGA, 2021; CUNHA, 2021; POTONYACZ, 2021; MORIYA, 2021).
According to the website Portal Educação (2013), the procedure described in the previous paragraph can be performed by a health professional, who with one hand presses the BVM bag and with the other he holds the mask on the patient, however, this procedure can not ensure better ventilation of the patient, therefore normally the BVM is operated by two healthcare professionals, one to hold the mask and monitor the patient and the other to press the BVM bag with both hands. However, both forms can harm the patient, as, according to Oliveira et al. (2011), “inadequate compressions can cause hyperventilation, barotrauma and reduced cardiac output”, so, to reduce this risk, all BVM’s have pressure regulator valves that limit the pressure to a maximum of 45 cmH2O. Thus, Oliveira et al. (2013) advises that “the MR should be used with the pressure regulator valve unlocked and the pressure of the device monitored with a manometer”.
As can be seen, the development of BVM-based mechanical ventilators can be complex, as the patient’s life can depend exclusively on this equipment, where the patient can stay connected to it for days. Therefore, they have complex volumetric and pressureometric control technology, and that, for this and other reasons, their development requires numerous steps and tests, which may take up to 2 years to be produced by commercial manufacturers, according to Suzumura et al. (2020, p. 449), which adds that,
quando o ventilador mecânico somente fornecer ventilações em modo controlado, os pacientes que apresentam esforço (drive respiratório), com sedação leve ou sem sedação, ou aqueles em desmame da ventilação mecânica, além de estarem desconfortáveis e em assincronia com o equipamento, correm grande risco de eventos adversos graves, como lesão pulmonar induzida pela ventilação mecânica, barotrauma e piora das trocas gasosas, dificultando o processo para voltarem a respirar de forma autônoma, sem uso de equipamentos. Diante dessas condições clínicas, ventiladores alternativos, que não permitem sincronia com o paciente, não devem ser utilizados para suporte de pacientes críticos por tempo prolongado.
Thus, still according to Suzumura et al. (2020, p. 445), “one of the challenges for the development of alternative ventilators is the lack of knowledge about physiology, respiratory mechanics and mechanisms of lung injury induced by mechanical ventilation by professionals without training in the health area”. In this context, according to Tharion et al. (2020), it should be mentioned that, despite the many projects of rapid implementation of prototyped ventilators, which are currently being developed for use in this pandemic, based on the modification of a BVM bag with an automated compression mechanism, they could be a viable option , it must be considered that precise control of ventilator output volume is a critical requirement when ventilating an intubated patient, so the BVM bag design poses an issue. For, due to variations in physical shape (different designs between brands) in the available BVM bags, adjustments would have to be made to the mechanical system to accommodate the differences in them. According to Johar and Yadav (2020), these designs, although they are a cheap and easy way to force air into the patient’s lungs, are not suitable for use, since they can be fatal for the patient, resulting in barotrauma. Most low-cost ventilator designs are based on a robotic way of squeezing and releasing the BVM bag, which cannot accurately control inspiration pressure; therefore, there is a high chance of damaging the alveoli (very delicate tissues in the lung). Due to the difficulties pointed out in this paragraph, the authors cited here proposed the development of mechanical ventilators, which were not based on the BVM, using, for this purpose, an air compressor and oxygen cylinders, whose ventilatory support is monitored and controlled by a microcontrolled system.
The National Health Surveillance Agency (ANVISA)[8], following the trend in the production of ventilators based on MR automation, issued a Collegiate Board Resolution on May 15, 2020 (RDC 386/2020), which establishes standards for the manufacture, registration and donation of these types of equipment in Brazil, called by her “Emergency and Transitory Respiratory Support Equipment type ‘Automated Ambu’” (BRASIL, 2020). According to the guidelines contained in this RDC, ANVISA recommends that first tests be carried out on benches and that, in order to prove the clinical requirements and the indication for use of the equipment, these, according to Brasil (2020), “must be attested by clinical reports issued by at least two physicians, specialists in the areas of anesthesia, intensive care or pulmonology, with proven experience in intensive care”.
In view of all that has been exposed, professors and students from the Santos Dumont campus, in addition to an external collaborator in the health area, authors of this work, in an extension project, approved by Public Notice 06/2020 – Selection of strategic and emergency extension projects for actions to combat and disseminate information regarding Covid-19 – from the Instituto Federal do Sudeste de Minas Gerais, developed an Electromechanical System (ES) system for automatically pressing the bag of the manual resuscitator. Thus, according to the work by Duarte et al. (2021), this research aims to offer equipment in which the manual resuscitator can be adapted and easily used by health professionals, in emergency and transient care for patients with breathing difficulties, when there are no ICU beds with artificial respirators available. In addition, the ES developed facilitates the use of the manual resuscitator, as the health professional does not need to keep pumping the bag with one hand and the other controlling the mask on the patient’s face. In this sense, the professional regulates the SE in the correct volume and frequency of pumping and has his attention more directed to the patient, reducing the effort undertaken in pumping, thus improving the professional’s working conditions and enabling more appropriate care for patients. Due to the difficulties and complexities presented in the previous paragraphs, for the development of a low-cost pulmonary ventilator based on BVM automation, the team opted for the development of a more simplified model in the expectation of creating a low-cost device, with rapid certification and validation by the regulatory agency, and it must be used exclusively by a trained health professional and under his constant supervision during the entire mechanical ventilation procedure, therefore, this equipment must be used in the same situations and conditions to which they are undergoing BVM, including under the same care (DUARTE et al., 2021).
3. MATERIALS AND METHODS
In this section, the methodologies and materials used to carry out the pre-clinical tests will be presented. Likewise, the methodology for composition of the bank, analysis and evaluation of test results will be presented.
3.1 MATERIALS FOR PRE-CLINICAL TRIALS ON WORKBENCHES
To carry out this study, the prototype developed by the authors of this work during the first phase of the extension project was used, according to Duarte et al. (2021), described in section 1. In this context, the prototype, to be submitted to this study, underwent corrections and improvements in relation to the previous version developed by Duarte et al. (2021), being represented in Figure 1.
Figure 1 – The prototype developed in previous work
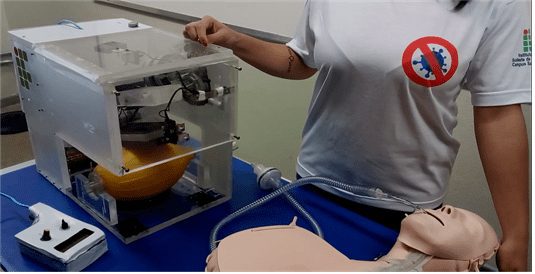
As can be seen in Figure 1, the prototype has a 15 mm acrylic structure and is mainly composed of: a mechanical arm and a cam-type mechanism for pressing the BVM bag; a brush motor type 12 V and 42 W, responsible for the movement of the arm; and 4 electronic circuit boards, one of which is PWM for controlling the torque when pressing, one for reversing the motor rotation, one for automatic switching between the 12 V power supply and the 12 V batteries of the new type break and one with a microcontrolled circuit based on the ATMEGA 328, responsible for controlling and monitoring the ventilation operation, including the operating time, the Breathing Intakes Per Minute (IRPM)[9], the audible alarm in case of operating failure and the battery alert weak. The prototype can also be controlled from a distance by remote control via cable (1.5 m), consisting of two potentiometers, one for controlling the intensity of pressure and the other for the time between breaths, LCD display that provides information such as operating time, IRPM and low battery level.
To carry out the pre-clinical tests on the equipment developed, the following were used: a 1600 ml adult Oxigel BVM device installed in the prototype; a test lung, to verify the correct ventilation provided by the prototype; an inflatable CPR dummy, used to simulate the patient; a 1.2 m breathing tube, used to connect the CPR dummy at a safe distance from the prototype; an HMEF filter; an endotracheal tube for intubation in the CPR dummy; a pressure calibrator from the Elomed brand, which is used to digitally measure the peak pressure provided by the ventilations; a liquid measuring cup, to measure the volume delivered in ventilations using water; a 30 cm school ruler, to measure the CPR dummy’s chest expansion during ventilations; a digital scale to register the final weight of the equipment; and a digital camera to record the tests.
3.2 METHODOLOGY FOR PRE-CLINICAL BENCH TESTS
Pre-clinical tests were carried out on a bench, in a controlled environment, using the prototype and the materials described in subsection 3.1, to demonstrate the proper functioning of the prototype in the care of patients with breathing difficulties. For the evaluation of the pre-clinical trials, the authors based themselves on the RDC 386/2020, described in section 2, where the trials were evaluated by a panel of judges composed of at least 5 (five) different health professionals, with the aim of objective of identifying the feasibility of using the prototype in the care of patients with breathing difficulties, indicating in which cases it is able to be used. Therefore, after the research was authorized by the Ethics Committee for Research with Human Beings (CEPH)[10] of the Instituto Federal do Sudeste de Minas Gerais, the committee was formed by: two specialists, as recommended by RDC 386/2020, an intensive care physician and an anesthetist. In addition to them, four other health professionals were invited by the development team, two nurses, a nursing technician and a physiotherapist. These last four were defined by the authors themselves, as they understand that the prototype can be used by different professionals, in different circumstances and for different types of diseases that involve breathing difficulties. Thus, all invited judges signed the Free and Informed Consent Form (TCLE)[11].
It should be noted that the entire process of developing the prototype, as well as testing and evaluating it, is not intended to follow exactly all the requirements of the RDC, since the Instituto Federal do Sudeste de Minas Gerais is not a company certified by the ANVISA for the manufacture of health equipment and because the evaluated device is not the final product to be marketed. However, it was decided to carry out the tests based on the criteria and procedures of this RDC, in order to obtain a better evaluation of the prototype and demonstrate its viability for which it was developed, thus enabling future partnerships with companies certified by ANVISA for manufacturing of equipment based on the prototype approved in the tests and evaluations.
In this context, the tests were carried out in two stages. In the first, tests with the prototype were carried out on a bench, in which data on frequency, volume, pressure and chest expansion of the CPR dummy were recorded to be later presented to the bench described above. In the second stage, the tests were carried out using the prototype to simulate the care of a patient with breathing difficulties, represented by a CPR dummy, under the supervision of the panel of judges, seeking to demonstrate to it, the usability, safety, ventilatory support and the feasibility of using the prototype in this type of service.
In this sense, in the first stage, four types of tests were performed, namely respiratory rate, inspired volume, inspiratory pressure and chest expansion. Thus, in all of them, the BVM was pressured both by a health professional, co-author of this work and external collaborator of the extension project, and by the developed prototype, for the purpose of comparing the results. So, these were also compared with the results of other works and published studies.
Thus, in all four types of tests, data were first recorded with the manual pressure of the BVM, performed by the health professional who chose three levels of pressure according to her experience in assistance with the BVM, identifying them as minimum, medium and maximum. After the tests with her pressing the BVM manually, the health professional inserted it into the prototype and adjusted it in order to obtain, approximately, the same three levels (minimum, medium and maximum), but now, with the pressure of the BVM performed automatically by the prototype, also registering the data. A better detail of the execution of each type of test is described below:
a) Breathing rate test
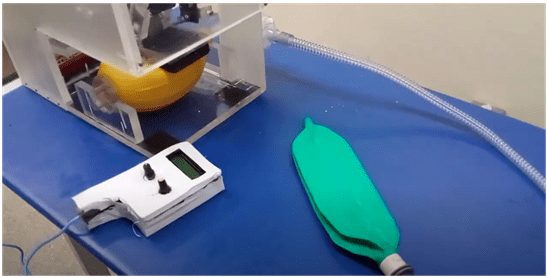
To perform the test of the frequency of Breaths Per Minute (BPM) with the prototype, we tried to record the test data with all the frequencies recommended in RDC 386/2020, from 8 BPM to 40 BPM, for each stipulated volume level previously (minimum, medium and maximum). For this, the prototype, the BVM, the 1.2 m trachea, the HMEF filter, the test lung and the digital camera were used to record the tests, according to the scenario reproduced in Figure 2.
Figure 2 – Scenario of respiratory rate tests with the prototype
b) Inspired volume test
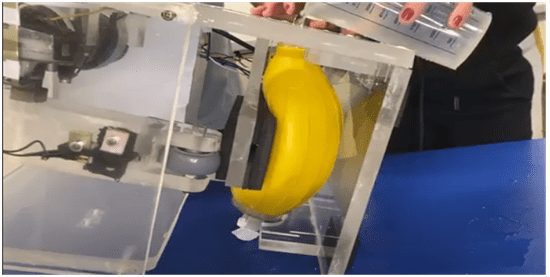
To carry out the inspired volume tests, the prototype, the BVM, a liquid and water measuring container was used. First, the health professional pressed the BVM to a minimum level, with it in the vertical position, that is, with the air outlet facing upwards, and remained with it pressed while another co-author of the work filled the BVM bag with water , until it was full. Then, the water was poured into a measuring container and the value of the volume obtained was recorded. So, this procedure was also performed on the other two levels, pressing with one and two hands. Finally, the same procedure was repeated, but now with the prototype pressing.
In this case, first the pressure on the BVM was stopped with the mechanical arm in the position desired by the professional (minimum, medium or maximum) and it was turned off; The equipment was placed in a vertical position with the air outlet facing upwards, it was filled with water, the water was poured out and the value of the volume obtained was recorded, and this procedure was repeated for the other two levels. In this way, the oxygen inlet was blocked to prevent the water outflow. This test scenario is reproduced in Figure 3.
Figure 3 – Inspired volume testing scenario with the prototype
c) Inspiratory pressure test
For the inspiratory pressure test, an Elomed pressure gauge was used, whose input was connected between the 1.2 m trachea and the test lung. In addition to these, the HMEF filter, a BVM and the prototype were used, and the measurements were recorded with a camera. In this context, the health professional connected the BVM and the HMEF filter to the other end of the trachea and manually pressed the BVM bag, for a few times, at the mentioned levels with a respiratory rate around 20 BPM, while another co-author recorded the Inspiratory pressure values displayed on the pressure gauge display using the camera. Then, the same tests were carried out with the equipment pressing the BVM, where the professional inserted it in the prototype and adjusted the equipment at the mentioned levels, while another co-author of this work recorded the obtained inspiratory pressure values. This test scenario is reproduced in Figure 4.
Figure 4 – Scenario of inspiratory pressure tests with the prototype

d) Chest expansion test
For the chest expansion tests, the CPR dummy, the 30 cm ruler, the 1.2 m trachea, the HMEF filter, the endotracheal tube, the BVM and the prototype were used. The ruler was supported on the table and positioned in front of the CPR dummy’s chest, in order to measure chest expansion, as shown in Figure 5.
Figure 5 – Scenario of chest expansion tests with the prototype and CPR dummy
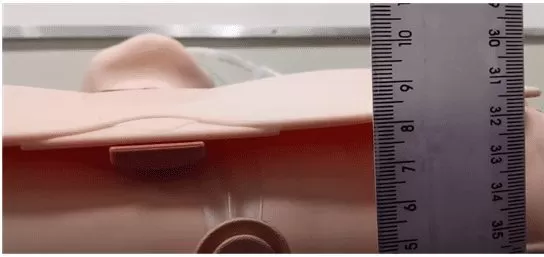
In this way, the health professional pressed the BVM manually on the 3 levels, one at a time, for a few times, with a frequency of around 20 BPM and recorded the values in cm of the elevation obtained. Then, the procedure was repeated now with the prototype pressing automatically and, after adjusting the pressing at the stipulated levels, the values obtained in cm of elevation were recorded.
That said, the second stage involved presenting the prototype and its functionalities to the panel of judges, through a test in which care for a patient with breathing difficulties was simulated. In this sense, the demonstration of the functionalities took place as follows: in the developed prototype, the health professional who co-authored this work, inserted the BVM device in its own compartment and prepared it for the service, connecting the 1.2 m long trachea , the HMEF filter, the mask or endotracheal tube as needed, the Manometer and the PEEP Valve, in some cases.
Next, care for a patient with breathing difficulties was simulated, represented here by a CPR dummy, using the prototype and demonstrating its usability, safety, ventilatory support and viability for this type of care to the bank. To do this, she connected the prototype to the electrical network (it could also be used by the battery if applicable), placed it close to the patient, but at a safe distance (according to the length of the trachea), positioned herself in front of the patient to place the mask or tube in it and pressed the on button to start the prototype working.
From that moment on, he controlled the respiratory incursions (frequency and volume) through the remote control, which, in turn, could be placed next to the patient’s head and close to the hands of the health professional, operator of the equipment, allowing her to hold the mask on the patient. Thus, the health professional demonstrated at least three levels of ventilation, as done in the bench tests, applying frequency, pressure and minimum, medium and maximum volumes to the patient and also simulating a lack of energy and continuity of operation of the prototype by the batteries. In this way, the simulation demonstrated that it is easier for the health professional to make the necessary adjustments in the respiratory incursions, either according to the signs of cyanosis described in section 2, or by the information displayed on the remote control display, or even by the observation of pressure on the BVM bag performed by the mechanical arm of the prototype, which, being transparent, facilitates the visualization, for example, of the force applied by it on the bag.
The health professional then monitored the patient’s evolution until he was able to breathe on his own again or was transported to a hospital with adequate resources, such as an artificial respirator, for example. After the service, she demonstrated that the BVM device could be easily removed from the equipment and both could be cleaned.
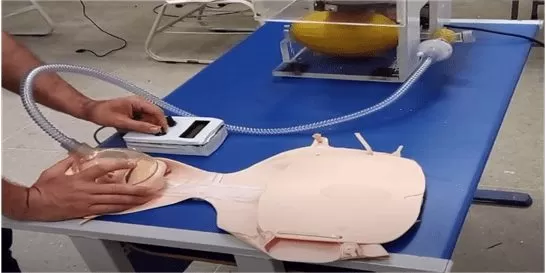
Thus, throughout the care, the authors recommend the presence of a health professional to monitor the patient, as the prototype does not have monitoring systems. So, the simulation is performed according to the example scenario reproduced in Figure 6.
Figure 6 – Simulating care for a patient with breathing difficulties
3.3 METHODOLOGY FOR ASSESSING THE RESULTS OF TESTS AND DEMONSTRATIONS
The evaluation of the test results of the first stage and the demonstrations of the second stage took place after the completion of the latter. Thus, after the demonstrations described in the previous paragraph, the test results of the first stage were presented to the bank, in addition to the videos of the tests for further analysis, if they so desired. Then, each member of the panel, individually, filled out a form for evaluating the results and demonstrations for each of the five evaluated categories, with at least 9 propositions in each one, these categories being: usability, which aims to evaluate how much the prototype is easy to use; safety, which aims to assess whether the prototype is safe and does not pose risks, both for the health professional and for the patient during handling; electrical safety, whose objective is the same as the previous category, in addition to assessing whether it works in the event of a power outage; mechanical ventilation, which aims to assess whether the device provides adequate ventilatory support at each level of respiratory difficulty, especially in relation to pressure and volume; and, finally, feasibility, which aims to assess whether, in view of all that has been exposed, it can be used to care for patients with different types of breathing difficulties, including COVID-19.
Lastly, each member of the committee issued a clinical report, where he was exposed in which situations it is able to be used, in addition to criticisms and suggestions for improvement. In addition, the results of the pre-clinical bench tests were compared with those of other related works, described in section 2, and with the results of the work by Ortiz et al. (2013), in which they evaluated the main BVM’s used in Brazilian ICUs with the functional requirements recommended by ANVISA’s RDC 386/2020.
4. RESULTS AND DISCUSSION
4.1 PRESENTATION OF THE RESULTS OF THE PRECLINICAL TRIALS
The results of the first stage of tests, carried out on a bench, demonstrated a good proximity between the inspired volume, inspiratory pressure and chest expansion values provided by both the manually pressed BVM and the automatically pressed BVM by the prototype. However, there were some variations between the values provided with the use of the prototype, as these were adjusted by the professional until reaching the stipulated levels of barotrauma in the tests, so that lower, higher or closer values are possible, depending on the adjustment of the prototype. Thus, the mean values of inspired volume, inspiratory pressure and chest expansion recorded in the tests, both with the BVM bag pressed manually by the health professional and with it automatically pressed by the prototype, can be seen respectively in Tables 1 and 2.
Table 1 – Results of the tests of inspiratory pressure, inspired volume and chest expansion with pressure on the BVM bag performed by the health professional with both hands
| Test type | Minimum | Medium | Maximum |
| Inspired volume | 50 ml | 400 ml | 700 ml |
| Inspiratory pressure | 7,24 cmH2O | 15,78 cmH2O | 23,94 cmH2O |
| Chest expansion | 8,16 cm | 8,56 cm | 9,56 cm |
Source: From the Authors (2021).
Table 2 – Results of the tests of inspiratory pressure, inspired volume and chest expansion with pressure on the BVM bag performed by the prototype.
| Test type | Minimum | Medium | Maximum |
| Inspired volume | 100 ml | 300 ml | 550 ml |
| Inspiratory pressure | 8,23 cmH2O | 15,76 cmH2O | 28,28 cmH2O |
| Chest expansion | 8,15 cm | 8,45 cm | 9,66 cm |
Source: From the Authors (2021).
A better description of the results reproduced in Tables 1 and 2 is described below:
a) Results of inspired volume tests
Inspired volume tests were performed as described in subsection 3.2. Therefore, first, the total volume of the BVM bag was identified, where it was completely filled with water, recording a volume of 1550 ml. After recording the total volume of the BVM bag, it was emptied and then the health professional pressed it with both hands, simulating a minimum pressure, and kept it pressed; the bag was again filled with water and after subtracting the total and current volumes, an inspired volume of 50 ml was recorded. After recording the volume at a minimum pressure, the same procedure was performed, but now simulating a medium pressure, with an inspired volume of 400 ml being recorded. Following the same procedure, the health professional pressed the BVM bag to the maximum with both hands and a volume of 700 ml was recorded. In the last volume test with manual pressure performed by the health professional, the only volume test with pressure with one hand was performed, where an inspired volume of 600 ml was recorded. After carrying out the tests with the health professional pressing the BVM bag manually, the same types of tests were started with the prototype automatically pressing the BVM bag, and according to the procedure explained in subsection 3.2, the inspired volumes recorded in a minimum pressure were of 100 ml, medium of 300 ml and maximum of 550 ml.
b) Results of the inspiratory pressure tests
After carrying out the inspired volume tests, the inspiratory pressure tests were started, following the procedures and using the materials described in subsection 3.2. In view of this, first, the BVM bag pressure tests were carried out manually by the health professional, at the three levels (minimum, medium and maximum) and with both hands. Thus, in 12 pressures of the BVM bag at a minimum level, the average of the recorded minimum pressure values was 7.24 cmH2O. Now, in 12 pressures of the BVM bag at a medium level, the average of the mean pressure values recorded was 15.78 cmH2O and finally in 9 pressures of the BVM bag at a maximum level, the average of the maximum pressure values recorded it was 23.94 cmH2O. Thus, after carrying out the pressure tests carried out manually by the health professional, the same tests were started, but now, with the BVM bag being automatically pressed by the prototype also at the three levels (minimum, medium and maximum), adjusted in the control of the same by the health professional. Thus, in 7 pressures of the BVM bag at a minimum level, the average of the recorded minimum pressure values was 8.23 cmH2O. Already in 7 pressures of the BVM bag at a medium level, the average of the recorded pressure values was 15.76 cmH2O and finally in 10 pressures of the BVM bag at a maximum level, the average of the maximum pressure values recorded was at 28.28 cmH2O.
c) Results of chest expansion tests
After performing the pressure tests, the CPR dummy chest expansion tests were started, following the procedures and using the materials described in subsection 3.2. The observed and registered values started from 8 cm, as this is the size of the chest of the CPR dummy when it is at rest. Therefore, this measurement was made with a 30 cm vertical school ruler, with its end resting on the table and parallel to the puppet’s chest. In this context, first, pressure tests on the BVM bag were carried out manually by the health professional, recording the three levels of expansion of the CPR dummy’s chest (minimum, medium and maximum), with both hands and in 6 repetitions by level. Thus, at a minimum level, the average of the chest expansion values recorded was 8.16 cm. Already at a medium level, the average of the chest expansion values recorded was 8.56 cm and finally at a maximum level, the average of the chest expansion values recorded was 9.66 cm. After carrying out the chest expansion tests with pressures on the BVM bag performed manually by the health professional, the tests were started with the BVM bag automatically pressed by the prototype also at the three levels (minimum, medium and maximum), adjusted in the control of the same by the health professional, also with 6 repetitions per level. Thus, at a minimum level, the average of the chest expansion values recorded was 8.15 cm. Already at a medium level, the average of the chest expansion values recorded was 8.45 cm and finally at a maximum level, the average of the chest expansion values recorded was 9.66 cm.
d) Results of respiratory rate tests
In tests of Breaths Per Minute (BPM) with the prototype, although it could be adjusted to different frequencies, it was easily adjusted to work at 8, 10, 15, 20 and 30 BPM, in the three stipulated pressure levels. In this way, in these tests, the prototype achieved satisfactory performance, however, to work at a frequency of 40 BPM, it was only possible to reach it at the maximum volume level provided.
4.2 COMPARISON OF TESTS RESULTS TO OTHER WORKS
As seen in subsection 4.1, the prototype can provide adequate ventilatory support to each patient in the same way when operated manually by the professional. And this can also be proven when comparing the maximum volume provided by the prototype (550 ml) with the results obtained in a study by Ortiz et al. (2013), who evaluated eight brands of BVM used in Brazilian ICUs and concluded that most of them provide a total volume of less than 600 ml when operated by different health professionals, using one hand. However, according to the authors, this value is lower than that suggested by the ASTM (American Society for Testing and Materials), which recommends a total volume greater than or equal to 600 ml. In this context, Ortiz et al. (2013) recommend that these BVM’s be handled with both hands, when higher volumes are desired.
In the case of the prototype, values higher than this can be achieved by making small adjustments to its mechanical arm. The tested respiratory rate values demonstrate proximity to the operating frequency range of the work by Chauhan et al. (2021), which works between 8 and 30 BPM. On the other hand, the provided volume results, average and maximum, operate within the range of values provided by the prototypes developed in the work of Al Husseini et al. (2010), Domènech-Mestres et al. (2021), Vasan (2020) and Chauhan et al. (2021), which vary on average from 200 ml to 800 ml. On the other hand, the delivered or inspiratory pressure results show proximity to the operating range of Vasan’s work (2020), around 10 to 30 cmH2O.
Finally, the frequency, volume and pressure results obtained in the bench tests are in accordance with the operating ranges recommended by RDC 386/2020 as functional requirements, which are respiratory rate between 8 and 40 BPM, inspired volume between 50 and 700 ml, and pressure in the inspiratory phase (delta) between 5 and 30 cmH2O.
The weight of the prototype with the BVM installed was 17.6 kg, which demonstrates the need to reduce weight to make it easier to transport. The final cost of the prototype was around R$ 2,000.00, a high value when compared to the prototypes developed in the works of the authors mentioned in section 2, but which could be reduced with a review of the materials used and with the production on a large scale.
4.3 EVALUATION OF RESULTS AND STATEMENTS BY THE JUDGES’ BOARD
As described in subsection 3.3, the second stage of testing was performed with a demonstration of the prototype’s functionalities, simulating care for a patient with breathing difficulties under the supervision of the panel of judges. After the demonstrations, the results described in subsection 4.1 were presented to the bank that, finally, carried out the evaluation of the results of both stages by filling out five forms, one for each category evaluated, in addition to reports in which they indicated the indication of use of the prototype, as described in subsection 3.3. The raw data collected from the forms completed by the judges were organized into spreadsheets for better analysis. In this way, each evaluated category and its results will be presented and discussed below:
a) Analysis of the usability evaluation of the prototype by the panel of judges
In evaluating the usability category, the six Judges analyzed 15 propositions, in which they considered the prototype easy to use by the health professional in the care of patients with different types of breathing difficulties. Then, 90 evaluations were carried out in this category, in which in 88 (97.78%) of them the judges agreed that the project is easy to use, and in 70 (77.78%) they completely agreed and in 18 ( 20%) partially agreed, as shown in Figure 7.
Figure 7 – Judges’ opinion regarding the usability of the prototype in the care of patients with breathing difficulties
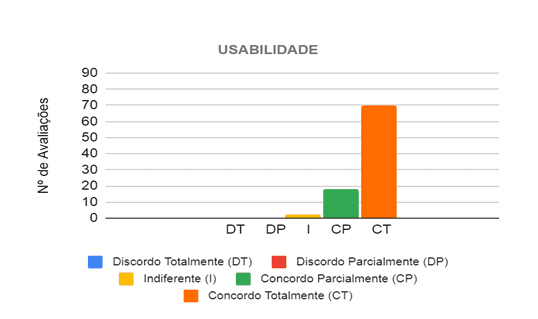 Source: From the Authors (2021).
Source: From the Authors (2021).
Thus, the main propositions in which, at least, five of the six judges fully agreed, concern the operationalization of the prototype, such as ease of removing and placing the BVM in the prototype for maintenance and cleaning, identifying its functionalities and being operated by only one professional of health. There were no propositions in which the judges disagreed, however, the three propositions that they did not totally agree with are related to its weight and size, where it can be verified that the prototype is not so easy to be transported, requiring improvements in the final design to in order to reduce your weight mainly.
b) Analysis of the prototype safety assessment by the panel of judges
In evaluating the safety category, the six judges analyzed nine propositions, in which they considered that the prototype is safe to be used in the care of patients with different types of breathing difficulties. Then, 54 evaluations were carried out in this category, in which in 50 (92.59%) of them the judges agreed that the project is safe, and in 42 (77.78%) they completely agreed and in 8 (14.81 %) partially agreed, as shown in Figure 8.
Figure 8 – Judges’ opinion regarding the safety of the prototype in the care of patients with breathing difficulties
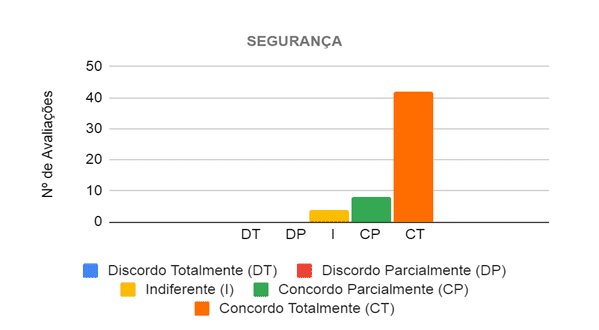 Source: From the Authors (2021).
Source: From the Authors (2021).
Thus, the main propositions in which at least five of the six judges completely agreed, considered a low risk of accidents for the health professional when handling the prototype, due to the protections and indications of the moving parts, as well as, not having sharp parts and or piercing. There were no propositions in which they disagreed, however, those that divided the opinions of the evaluators and were not so well evaluated are related to the fact that propositions are not sure, after the prototype is in operation, if there is no risk of the BVM leave the initial position, if it can stop working accidentally and if it does not offer risks to the patient. In this way, some judges informed, through the observation field of the form, that in order to properly judge these propositions, it would be necessary to test it in the field.
c) Analysis of the electrical safety assessment of the prototype by the panel of judges
In the evaluation of the electrical safety category, the six judges analyzed eight propositions, in which they considered the prototype electrically safe in its operation and in its use by the health professional when caring for patients with different types of breathing difficulties. Then, 48 evaluations were carried out, in which in 46 (95.83%) of them the judges agreed that the project is electrically safe, and in 43 (89.58%) they completely agreed and in 3 (6.25% ) partially agreed, as shown in Figure 9.
Figure 9 – Judges’ opinion regarding the electrical safety of the prototype in the care of patients with breathing difficulties
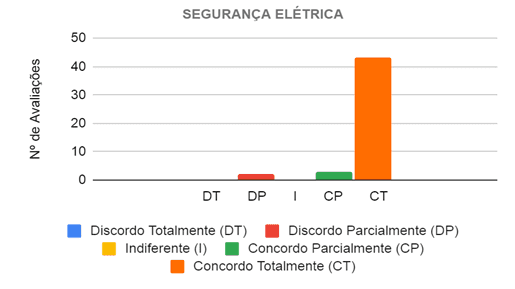 Source: From the Authors (2021).
Source: From the Authors (2021).
Thus, there was only one proposition that divided the opinion of the evaluators, where two (33.33%) of the evaluators disagreed and two (33.33%) partially agreed that there are no apparent wirings and are not at risk of breaking, that is, the prototype needs improvements to hide the few wires that were still showing.
d) Analysis of the assessment of ventilatory support provided by the prototype by the panel of judges
In evaluating the mechanical ventilation category, the six judges analyzed 11 propositions, in which they considered that the prototype provides adequate ventilation, depending on the case, to the patient attended. Sixty-six evaluations were then carried out in this category, in which in 60 (90.91%) the judges agreed that the project provides adequate ventilation, and in 48 (72.73%) they completely agreed and in 12 (18.18 %) partially agreed, as shown in Figure 10.
Figure 10 – Judges’ opinion regarding the ventilatory support provided by the prototype in the care of patients with breathing difficulties
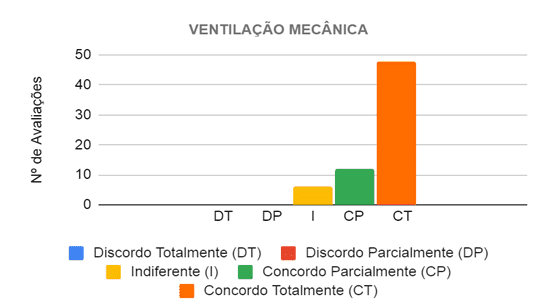
Thus, the main propositions in which at least five of the six judges completely agreed state that the prototype provides adequate ventilation both in terms of volume and pressure to patients with mild and moderate breathing difficulties. There were no propositions in which they disagreed, however, those that divided the opinions of the evaluators, where three of them did not totally agree, are related to the ventilation provided to patients with severe breathing difficulties, both in terms of volume and pressure. Thus, some judges informed, through the observation field of the form, that in order to properly judge these propositions, it would be necessary to test it in patients.
e) Analysis of the feasibility assessment of the prototype by the panel of judges
In the evaluation of the feasibility category, the six judges analyzed 11 propositions, in which they considered that the prototype is viable for the care of patients with different levels of respiratory difficulty. Sixty-six evaluations were then carried out, in which in 55 (83.33%) the judges agreed that the project is viable, and in 42 (63.64%) they completely agreed and in 13 (19.70%) they agreed partially, as shown in Figure 11.
Figure 11 – Judges’ opinion regarding the viability of the prototype in the care of patients with breathing difficulties
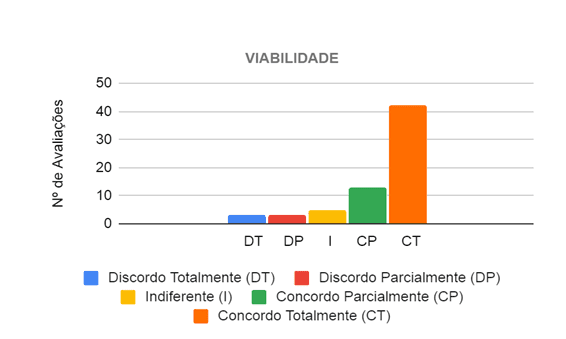
Thus, the main propositions in which at least five of the six evaluators fully agreed, state that the project is viable in the care of patients with mild and moderate breathing difficulties, as well as patients with Cardiorespiratory Arrests (CRA), in addition to favoring the working conditions of the health professional and allow for better patient care by this professional, since he does not have to press the BVM bag, thus being able to dedicate greater attention to the patient. In this context, only the propositions related to COVID-19 divided the opinions of the evaluators, where 2 (33.3%) of them disagreed, 3 (50%) agreed and 1 (16.7%) was indifferent as to whether the project was viable for care for patients with COVID-19. So, some evaluators reported, through the observation field of the form, that to properly judge these propositions it would be necessary to test it on patients and observed the risk of contamination with the use of the prototype even using protection filters.
f) Analysis of the clinical reports issued by the panel of judges
Finally, after assessing the demonstrations and test results, the evaluators made a final assessment by issuing individual clinical reports, pointing out final considerations and indicating the use of the prototype when in its final version produced by a company authorized by the ANVISA. In this way, the intensive care physician, the anesthetist and the other four health professionals (two nurses, a nursing technician and a physiotherapist), as recommended by RDC 386/2021, issued clinical reports. Of these, the intensive care physician reported that the project in its final version is indicated for the care of patients with acute respiratory failure, mechanical pre-ventilation and CRA. The anesthetist, in turn, indicated it to replace the repetitive effort of the health professional, providing greater comfort and ergonomics to the same and, consequently, better monitoring of the patient’s ventilation. So, the nurse and the nursing technician considered that, after making the improvements regarding the decrease in weight and size and the increase in the duration of the battery, the final version of this project will be of great help in patient care with breathing difficulties, mainly because it is easy to use, avoids professional wear and tear when using the BVM and allows for better patient ventilation. The physiotherapist, on the other hand, indicated its use in ambulances, emergency rooms and emergency rooms, always under the close supervision of the health professional, especially in relation to pressure, since, in some specific diseases, the lung is more rigid. And, finally, the nurse indicated the test in human patients for a better evaluation.
That said, an important point that should be taken into account was highlighted by the anesthesiologist in the clinical report, who recalled that the BVM device is still used manually due to the fact that many health professionals prefer it that way, as they can feel, through the contact of the hands with the BVM bag, how the patient’s lungs are and adjust ventilation better. But both the judge who made the observation and the health professional who accompanied the development and carried out the tests, stated that the fact that the structure of the prototype developed by the authors of this work is in transparent acrylic, minimizes the lack of this perception, since it is possible to observe how the mechanical arm acts on the BVM bag, as well as the force exerted by it on it, allowing the health professional to have an idea of how the patient’s lung may be and adjust adequate ventilation.
5. CONCLUSIONS
In order to answer the guiding question: is the development of low-cost artificial respirators based on the automation of manual resuscitators feasible for the care of people with respiratory impairment? This work aimed to evaluate the feasibility of the prototype developed at the Santos Dumont campus, at the Instituto Federal do Sudeste de Minas Gerais, in emergency care for patients with breathing difficulties. For this purpose, pre-clinical tests were carried out on the bench of the prototype of an electromechanical system for automating the pressure of the bag of a manual resuscitator and its evaluation by a panel of judges, composed of health professionals, based on the recommendations of the RDC 386/2020 of ANVISA.
Thus, according to the analysis of the results of the pre-clinical tests, it was verified that the prototype, automatically pressing the bag of the manual resuscitator, provides ventilatory support (respiratory rate, inspired volume and inspiratory pressure) very close to that provided by the health by manually pressing. In addition, with the analysis of the data collected by the forms applied to the judges and the clinical reports issued by them, after evaluation, it was verified that in more than 90% (295) of the 324 evaluations carried out, the judges agreed that the prototype is easy easy to use, safe, provides adequate ventilation in each case, and is feasible in caring for patients with different types and levels of breathing difficulties.
However, it is necessary to make some improvements in its structure in order to reduce its weight and size to improve its portability and consequently its cost. It is concluded, then, that the prototype is able to be produced and tested on human beings, therefore, the project will be made available to companies that comply with the requirements of ANVISA’s RDC 386/2020 and want to partner with the Santos Dumont campus, from the Instituto Federal do Sudeste de Minas Gerais, for donation to hospitals and emergency and urgent care units in the region.
REFERENCES
BRASIL. Covid em Manaus: sem oxigênio, pacientes dependem de ventilação manual para sobreviver em Manaus. BBC News Brasil, São Paulo, p.1, 15 de jan. de 2021. Disponível em: https://www.bbc.com/portuguese/brasil-55674229. Acesso em: 1 de ago. de 2021.
BRASIL. Resolução – RDC Nº 386, de 15 de maio de 2020. Diário Oficial da União Brasília, p.1-7, 15 de maio de 2020, seção 1. ed. extra. Disponível em: https://www.in.gov.br/web/dou/-/resolucao-rdc-n-386-de-15-de-maio-de-2020-258335933. Acesso em: 22 de ago. de 2021.
CHAUHAN, R. et al. Prototyping of a Low-Cost Portable Ventilation Device for Health Care in Developing Countries. International Research Journal of Engineering and Technology (IRJET), [s. l.], v. 08, p. 1-7, 01 de jan. de 2021.
CUNHA, V. Reanimador autoclavável reservatório de silicone e pvc. Domax Ind. Com. Imp. Exp. Equip. Hosp. Eirelli – EPP, São Paulo, SP, 2021, 1 p. Disponível em: http://domax.ind.br/images/manuais/REANIMADOR-HAOXI.pdf Acesso em: 08 de ago. de 2021
DIMAVE. Entenda a importância do reanimador manual! Dimave Equipamentos Hospitalares, [S. l.], p.1, 29 de ago. de 2019. Disponível em: https://blog.dimave.com.br/entenda-a-importancia-do-reanimador-manual/. Acesso em: 27 de abr. de 2020.
DOMÈNECH-MESTRES C. et al. Design for the Automation of an AMBU Spur II Manual Respirator. Machines. 2021, p. 9-16, v. 9, n. 2, p. 45. DOI: 10.3390/machines9020045
DUARTE, L. B. C. et al. Desenvolvimento de um sistema eletromecânico para automatização do reanimador manual. III Simpósio de Ensino, Pesquisa e Extensão, 23 de fev. de 2021, 1 ed., p. 19-20. Disponível em: https://www.ifsudestemg.edu.br/santosdumont/institucional/pesquisa/acoes-e-programas/simposio-de-ensino-pesquisa-e-extensao/anais-simposio-2020.pdf/view. Acesso em 22 de ago. de 2021.
HIGA, L. A. Reanimador manual Mark IV – Ambu. Cirúrgica Fernandes Ltda, Santana de Parnaíba, SP, 2021, 6 p. Disponível em: https://site-cfernandes-imagens-produtos.s3-sa-east-1.amazonaws.com/REANIMADOR+MANUAL+MARK+IV+-+AMBU.pdf Acesso em: 08 de ago. de 2021.
HUSSEINI, A. M. Al. et al. Design and Prototyping of a Low-cost Portable Mechanical Ventilator. Proceedings of the 2010 Design of Medical Devices Conference, Minneapolis, p. 1-9, 13 abr. 2010. DOI: 10.1115/1.3442790.
IWASA, A. Reanimador manual (Ambú) Protec. Protec Export Ind. Com. Imp. Exp. Equip. Méd. Hosp. Ltda, Cotia, SP, 2021, 14 p. Disponível em https://protec.com.br/wp-content/uploads/2021/06/Manual_Reanimador-manual_8058_Versao09.pdf Acesso em: 08 de ago. de 2021.
JOHAR, H. S.; YADAV, K. DRDO’s Portable Low-Cost Ventilator: “DEVEN”. Trans Indian Natl. Acad. Eng., 15 de jul. de 2020, 5, p. 365–371. https://doi.org/10.1007/s41403-020-00143-5.
LOPES, A.; BRITO, S. Soluções nacionais improvisadas tentam suprir a falta de respiradores. Veja, [S. l.], p.1, 27 de abr. de 2020. Disponível em: https://veja.abril.com.br/tecnologia/solucoes-nacionais-improvisadas-tentam-suprir-a-falta-de-respiradores/ Acesso em: 08 de ago. de 2021.
MORIYA, J. G. Ressuscitador Manual Reutilizável Besmed. Besmed Health Business Corp., Taiwan, China, 2021, 6 p. Disponível em: https://docplayer.com.br/11719928-Ressuscitador-manual-reutilizavel-besmed.html Acesso em: 08 de ago. de 2021.
OLIVEIRA, P. M. N. Fatores que afetam a ventilação com o reanimador manual autoinflável: uma revisão sistemática. Rev Paul Pediatr. 2011, v. 29, n. 4, p. 645-55.
OPAS. Folha informativa sobre COVID-19. Organização Pan-Americana de Saúde, 2021. Disponível em: https://www.paho.org/pt/covid19. Acesso em: 01 de ago. de 2021.
ORTIZ, T. de A. et al. Avaliação de reanimadores manuais utilizados em UTIs brasileiras. Jornal Brasileiro de Pneumologia, 06 de set. de 2013, v. 39, n. 5, p. 595-603. https://doi.org/10.1590/S1806-37132013000500010
PORTAL EDUCAÇÃO. Técnicas de ventilação para a insuficiência respiratória. Portal Educação. São Paulo, p.1, 2 de abr. de 2013. Disponível em: https://siteantigo.portaleducacao.com.br/conteudo/artigos/enfermagem/tecnicas-de-ventilacao-para-a-insuficiencia-respiratoria/41803. Acesso em: 22 de ago. de 2021.
POTONYACZ, G. Reanimador pulmonar RWR. RWR Indústria e Comércio de Equipamentos para Eletromedicina Ltda, São Bernardo do Campo, SP, 2021, 3 p. Disponível em: https://www.rwr.com.br/manuais/Reanimador-pulmonar.pdf Acesso em: 08 de ago. de 2021.
SHAHID, M. Prototyping of Artificial Respiration Machine Using AMBU Bag Compression. In: International Conference on Electronics, Information, and Communication (ICEIC), IEEE. 2019, Auckland, New Zealand, p.1–6. DOI: 10.23919/ELINFOCOM.2019.8706360.
SUZUMURA, E. A. et al. Desafios para o desenvolvimento de ventiladores alternativos de baixo custo durante a pandemia de COVID-19 no Brasil. Rev Bras Ter Intensiva, [s. l.], v. 32, n. 3, p. 444-457, 12 jun. 2020. DOI: 10.5935/0103-507X.20200075.
THARION, J. et al. Rapid Manufacturable Ventilator for Respiratory Emergencies of COVID – 19 Disease. Transactions of the Indian National Academy of Engineering, (2020), 5, p. 373–378 https://doi.org/10.1007/s41403-020-00118-6.
TSUZUKI, M. S. G. et al. Respirador Mecânico VENT19. CBA, [s. l.], v. 2, n. 1, p. 1-6, 7 dez. 2020. DOI: 10.48011/asba.v2i1.1552.
VASAN, A. et al. MADVent: A low-cost ventilator for patients with COVID-19. Med. Devices Sens. 2020, p. 1-14, v. 3, e10106. DOI: 10.1002/mds3.10106
VIDALE, G. Coronavírus: Brasil tem 61.000 respiradores funcionando. É suficiente? Veja, [S. l.], p. 1, 27 mar. 2020. Disponível em: https://veja.abril.com.br/saude/coronavirus-brasil-tem-61-000-respiradores-funcionando-e-suficiente/ Acesso em: 22 de abr. de 2020.
VITALAIRE. O que é ventilação mecânica: Entenda o sobre o tratamento e quem precisa. VitalAire. São Paulo, p.1, 2021. Disponível em: https://www.paho.org/pt/covid19. Acesso em: 01 de ago. de 2021.
WALLACE, A. Coronavírus: como funcionam os respiradores e por que eles são chave na luta contra a covid-19. BBC News Brasil, São Paulo, p.1, 31 de mar. de 2020. Disponível em: https://www.paho.org/pt/covid19 . Acesso em: 01 de ago. de 2021.
APPENDIX – FOOTNOTE
6. Sistema Único de Saúde (SUS).
7. https://emergency-vent.mit.edu/
8. Agência Nacional de Vigilância Sanitária (ANVISA).
9. Incursões Respiratórias Por Minuto (IRPM).
10. Comitê de Ética em Pesquisa com Seres Humanos (CEPH).
11. Termo de Consentimento Livre e Esclarecido (TCLE).
[1] Master in Computer Science, Specialist in Technology in Computer Networks. ORCID: 0000-0003-1543-0487.
[2] Degree in Mathematics, Technician in Industrial Mechanics. ORCID: 0000-0001-8079-7707.
[3] Technologist in Information Technology Management, Technician in Electrotechnics. ORCID: 0000-0001-7040-7170.
[4] Bachelor’s Degree in Railway and Subway Engineering, Technician in Maintenance of Subway Railway Systems. ORCID: 0000-0001-6422-0393.
[5] Technical Specialization in Occupational Nursing, Technical Nursing. ORCID: 0000-0001-7061-8410.
Submitted: March, 2022.
Approved: May, 2022.