ORIGINAL ARTICLE
TESCAROLLO, Iara Lúcia [1]
, OLIVEIRA, Aratã Carvalho [2], FIORIN, Arielli Pereira Couro [3], NASCIMENTO, Mariana Ferreira [4]
TESCAROLLO, Iara Lucia. et al. Characterization of orodispersible films formulated with natural flavors. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 04, Ed. 10, Vol. 06, p. 05-17. October 2019. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/orodispersible-films, DOI: 10.32749/nucleodoconhecimento.com.br/health/orodispersible-films
ABSTRACT
The oral route is one of the most used for the administration of solid dosage forms because it is convenient, economical and easy to administer, however, it may present as limitations the difficulty of swallowing for some patients. Orodispersible films (ODF) constitute a pharmaceutical form, innovative, practical, versatile and easy to administer. They are characterized by thin, flexible polymeric films developed for oral drug administration and can overcome the swallowing difficulties associated with solid dosage forms such as tablets and capsules. In addition to the drug, orodispersible dosage forms have in their composition film-forming polymers, plasticizers, sweeteners, saliva stimulating agents, flavoring agents, coloring agents, stabilizers, thickeners, permeation enhancers and disintegrants. Sweeteners, flavors and flavorings give ODF a sweet taste and pleasant odor and can contribute to masking excessively bitter drugs. The present study aimed to develop and evaluate the physicochemical and organoleptic properties of ODF formulated with two different flavorings so that they can be used to deliver drugs with oral absorption potential. Two samples were produced using the essential oils of Sicilian lemon and tangerine. The samples were submitted to a preliminary stability test and evaluated in terms of appearance, color, odor, flavor, pH, disintegration time and average weight. The results revealed potential in the use of Sicilian lemon and tangerine essential oils in the development of ODF, however, in the preliminary stability study, very noticeable changes were observed for the samples stored under different temperature conditions. Although other more in-depth studies should be conducted in the characterization of the samples produced, the approach adopted in this study can contribute to the construction of a scientific basis for the development of preparations involving ODF.
Keywords: Biopolymers, essential oils, pharmaceutical technology.
1. INTRODUCTION
The oral route is one of the most used for the administration of solid dosage forms because it is convenient, economical and easy to administer, however, it may present as limitations the difficulty of swallowing for pediatric and geriatric patients. Orodispersible systems (SODs) were developed to overcome swallowing difficulties associated with solid dosage forms such as tablets and capsules (GHOSH et al, 2011; BALA et al., 2013). Thus, they began to gain popularity due to their advantages such as rapid disintegration and dissolution, self-administration, not requiring chewing and drinking water (ARYA et al., 2010). They also dissolve quickly when placed on the tongue (ORLU et al., 2017), allowing better drug absorption (HOFFMANN; BREITENBACH; BREITKREUTZ, 2014). This particular characteristic allows greater availability and efficacy of the drug (PREIS; PEIN; BREITKREUTZ, 2012).
One of the first oral films was developed by Pfizer, called Listerine®™ and was used as a mouthwash to combat bad breath. The formulation of this type of film involves the use of film-forming polymers, plasticizers, different drugs, sweeteners, saliva stimulating agents, flavorings, dyes, stabilizers, thickeners, permeation enhancers and disintegrants (BALA et al., 2013). Sweeteners give the film a sweet taste and flavorings give a pleasant odor. Flavorants are also used to correct, at the same time, the taste and odor of formulations as well as to improve or mask the odor and unpleasant taste of some drugs (HOFFMANN; BREITENBACH; BREITKREUTZ, 2014).
Recently, several mucosal dosage forms have been proposed, including orodispersible films (ODF), adhesive tablets and gels (PREIS; PEIN; BREITKREUTZ, 2012; BALA et al., 2013). The promising role of ODF is to improve the delivery of drugs with different pharmacological actions. Studies indicate that ODF formulated with natural and biocompatible polymers such as cashew gum and gellan gum have been shown to be quite viable for insulin administration, with the advantages of reducing the inconveniences of enzymatic degradation of the drug in the digestive tract and low intestinal permeability (RIBEIRO et al. al., 2017). Other films based on pure gelatin and the binary mixture between starch and gelatin proved to be viable alternatives in the development of ODF for the release of active principles in the oral cavity (PEREIRA et al., 2019). The incorporation of local anesthetics such as prilocaine and lidocaine hydrochlorides in hydrophilic polymeric films was studied by Couto (2015). From this perspective, mucoadhesive films are presented as a non-invasive alternative in the administration of oral anesthetics with advantages in cost reduction, patient compliance, ease of application and lower risks of contamination and intoxication. Another study demonstrated that it was possible to formulate ODF with dicyclomine hydrochloride with the intention of obtaining better therapeutic efficiency with increased bioavailability and improved patient compliance. The plasticizer used polyethylene glycol (PEG-400) resulted in better films in relation to the evaluated physical-chemical parameters. Aspartame was used as a sweetener masking the bitter taste of the drug (TOMAR et al., 2012). In addition to the pharmaceutical area, the use of dispersible polymeric films has been studied in the cosmetic area (SANFELICE et al., 2010).
As described by Resta and Mali (2019) ODF formulations can be produced by the casting process (solvent evaporation). This process involves mixing the polymer in solution with a substrate, followed by evaporation of the solvent, which provides molecular orientation of the polymer molecules resulting in film formation (DENG et al., 2018). Depending on the thickness and size of the film formed after drying, the dose of the drug or bioactive that can be carried is determined (MUSAZZI et al., 2018)
There are some difficulties in the development of ODF, for example, high doses of drugs are not easily incorporated into film formers, there are several technical limitations such as the need to standardize the film thickness to ensure dose uniformity. The choice of packaging type is also important, as ODF are relatively fragile, requiring protection against moisture and high temperatures to prevent their disintegration. For excessively bitter drugs, masking the unpleasant taste becomes a major challenge. ODF can induce self-medication (NAND et al., 2010). The most common problems of ODF are related to their instability in environments with high relative humidity, the small dose of drug that can be incorporated, essentially due to their small size, low weight and thin thickness. Drugs that are unstable in oral pH or that can irritate the oral mucosa should not be formulated in this type of system (KESHARI; KUMAR SHARMA; PARVEZ, 2014).
The present study aimed to develop and evaluate the physicochemical properties of ODF formulated with two different natural flavorings so that they can be used to deliver drugs with oral absorption potential.
2. METHODOLOGY
2.1 MATERIAL
For the development of the formulations, different raw materials were used: Xanthan gum; Carrageenan Gum, Potassium Sorbate; Sodium benzoate; Acesulfame k; sucralose; Soy lecithin; Mannitol; Polysorbate 80; Propylene glycol; Simethicone 30%; Pullulan; Polyethylene glycol 400, purified water and the essential oils of Sicilian lemon (Citrus limon) and tangerine (Citrus reticulata).
The equipment used in the production and evaluation of the formulations were: Semi-analytical balance (Gehaka® brand, model BG440); digital pH meter (Gehaka® brand, model PG 2000); Laminator (Matrix® brand, model BF-FOX MV9102548-6); Glass plate (Brand Binder® KBF-115); Greenhouse (Gehaka® brand, model G-4023D); Air heater (Mondial® brand, model A-08); Guillotine (Maped Universal® brand, model G3208).
2.2 PREPARATION OF ORODISPERSIBLE FILMS
The ODF were produced by the casting technique, in which the film-forming solution was prepared in an aqueous medium, placed on a glass support for dehydration, and after evaporation of the solvent, the film was removed by detachment. Two formulations were elaborated, varying the type of flavoring used. For the development of the F1 sample, different raw materials were used, such as: Xanthan gum: 0.05 to 0.07%; Carrageenan Gum: 0.35 to 0.40%, Potassium Sorbate: 0.1 to 0.3%; Sodium benzoate: 0.05 to 0.15%; Acesulfame k: 0.2 to 0.5%; Sucralose: 0.1 to 0.3%; Soy lecithin: 0.5 to 1.0%; Mannitol: 0.1 to 0.3%; Polysorbate 80: 0.5 to 1.0%; Propylene glycol: 1.0 to 2.0%; Simethicone 30%: 0.5 to 1.0%; Pullulan: 15 to 20%; Polyethylene glycol 400: 1.0 to 2.0%, Sicilian lemon essential oil: 0.5% and purified water amount sufficient to 100%. For the development of sample F2, the same components were used, varying only in the flavoring Tangerine essential oil (0.5%).
Bench batches of 100g were produced in order to select the best formulation. Technique for preparing the orodispersible film base involved several steps adapted from studies published in the area (PEREIRA et al., 2019; RESTA and MALI, 2019; COUTO, 2015; FERREIRA et al., 2015).
The final production involved the following steps: Phase 1: Weigh separately the xanthan gum, carrageenan gum, pullulan, potassium sorbate, sodium benzoate, acesulfame k, sucralose, mannitol and soy lecithin, disperse in distilled water, homogenize and, in then let stand until completely dispersed. Phase 2: Separately weigh polysorbate 80, simethicone emulsion 30%, polyethylene glycol 400, propylene glycol, homogenize, then allow to stand until completely dispersed. Phase 3: After complete dispersion of Phase 1 and Phase 2, pour one phase into the other followed by homogenization. Phase 4: Add the flavorings. Phase 5: Disperse the mixture onto a glass plate, and distribute it evenly with the help of the laminator. Phase 6: Dry in an oven with air circulation at 30 °C for 8 hours. Stage 7: After drying, cut the film with the help of a guillotine to the standard size. Phase 8: Pack the films in appropriate packaging, separated by a parchment paper, incorporating a silica sachet to avoid moisture.
After preparing the films, they were evaluated in relation to quality tests in order to establish specifications such as appearance, color, odor, flavor, pH, disintegration time and average weight. The samples were also sent for the preliminary stability study. The trials were adapted from the Brazilian Pharmacopoeia (BRASIL, 2019) and studies developed by Sanfelice and collaborators (2010).
2.3 CHARACTERIZATION OF FILMS
The films were submitted to preliminary stability tests under different storage conditions: oven at 40º±2ºC; refrigerator at 5ºC±2ºC; ambient temperature protected from light (25º±5ºC) and ambient temperature with direct exposure to sunlight (25º±5ºC). The parameters evaluated were: pH; average weight; disintegration; taste, odor and appearance. The analyzes were performed at times zero (T0), and every 7 days, for a total of 28 days. The determination of the appearance, odor and flavor was carried out from the perception of the formulators, being considered a subjective parameter.
2.3.1 ASPECT
Each sample was transferred to a Petri dish, its appearance, color, homogeneity and uniformity were observed. The appearance was classified according to the following criteria: N (normal, without visible or perceptible alteration); LM (slightly modified); M (modified); MM (much modified).
2.3.2 ODOR
Each sample was transferred to the Petri dish, the odor was observed, which was characterized based on the type of flavoring used. The odor was classified according to the following criteria: N (normal, unaltered, characteristic of the type of flavoring used); LM (slightly modified); M (modified); MM (much modified).
2.3.3 FLAVOR
Each sample was transferred to the mouth and deposited under the tongue. After the experiment, the flavor was classified according to the following criteria: N (normal, unaltered, characteristic of the type of flavoring used); LM (slightly modified); M (modified); MM (much modified).
2.3.4 DETERMINATION OF pH
The determination of pH was performed using a potentiometer coupled to a pH-sensitive glass electrode. 10% solutions were prepared. Three consecutive readings were taken at room temperature.
2.3.5 DISASSEMBLY TIME
The disintegration test was performed as described by Perumal et al. (2008) also endorsed by Resta and Mali (2019). Phosphate buffer saline (pH 6.8) was used as a disintegration medium to simulate saliva. Samples were placed in 50 ml of phosphate buffer solution at 37°C and kept under agitation. The disintegration time was determined as the time (seconds) required for the film to disintegrate.
3. RESULTS AND DISCUSSION
The development of ODF requires the formulator to pay special attention to attributes related to organoleptic properties such as odor and taste. As described by Medeiros and Garruti (2018), most drugs have an intrinsically unpleasant taste and can provoke innate rejection reflexes in humans. A palatable drug is one in which the aversive sensory attributes have been minimized, masked or eliminated in its formulation. A flavoring is a natural or artificial substance that is added to the formulation to give or enhance the flavor and aroma, while the sweetener imparts a sweet taste to the preparation. The use of sweeteners and flavors is of fundamental importance to improve palatability, as they usually help mask the unpleasant taste of a product.
The search for new flavors is one of the trends in product development. In this study, two ODF were produced, varying only in the type of flavoring. In sample F1, 0.5% of Sicilian lemon essential oil was used and in F2, 0.5% of tangerine essential oil was used. Flavoring agents are very important in the production of oral pharmaceutical forms, and have the characteristic of intensifying not only the aroma but also the flavor. In general, it is important to use specific correctives or employ techniques to mask odor and taste to make the preparation palatable and increase patient compliance. The choice of flavoring or flavoring must be in accordance with the characteristics of the product (FERREIRA and BRANDÃO, 2008). In this study, Sicilian lemon essential oil and tangerine essential oil were used. These essential oils are among the most sold citrus compositions (blends) in the world for the cosmetic, drug, food and beverage industries (BIZZO; MARIA; REZENDE, 2009; BONACCORSI et al., 2011).
The production of ODF requires the choice of components that favor the formation of the film. In the development of samples F1 and F2, xanthan gum and carrageenan gum were used as stabilizers, thickeners, suspending and emulsifying agents. Potassium sorbate and sodium benzoate were used as preservatives. Components such as acesulfame k, sucralose and mannitol were used as soluble sweeteners. Soy lecithin and polysorbate 80 as emulsifiers, propylene glycol as humectant and simethicone 30% as defoaming agent. Polyethylene glycol 400 was used as a plasticizer and pullulan as a film-forming polymer (ROWE et al., 2009).
It is interesting to note that pullulan is a highly water-soluble polysaccharide, with great capacity to form films with considerable mechanical strength, is biodegradable and edible (KULKARNI et al., 2010; FERREIRA et al., 2015).
The ODF proposed in this study were flexible, with adequate rigidity, absence of cracks and bubbles, easy to disintegrate in phosphate buffer, pH ranging from 6.5 to 7.5 and pleasant organoleptic properties.
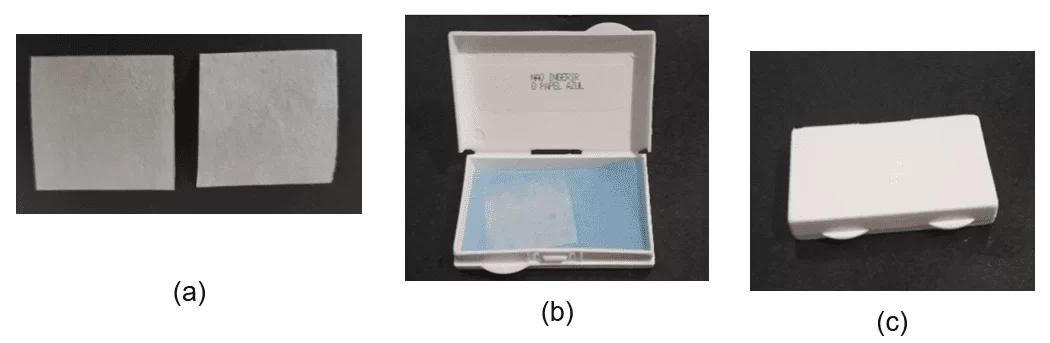
Figure 1 demonstrates the appearance of the individual films and their original packaging. The preparations presented characteristic odor and flavor of Sicilian lemon (F1) and tangerine (F2). Additionally, it is important to highlight that the flavor of both F1 and F2 was sweet. The average weight of 20 units measuring 3.0 cm 2 was 0.1156±0.0030 g for F1 and 0.1045±0.0025g and disintegration time less than 30 seconds (37ºC).
In the preliminary stability study, no changes were observed in the properties of ODF stored at room temperature and under exposure to indirect natural light during the entire study period T28 (28 days). Samples F1 and F2 were classified with the parameter N (normal, no visible or perceptible change). For samples stored at temperatures of 40º ± 2ºC and 5º ± 2ºC, important changes were observed from T7 (seven days). Both samples F1 and F2 were classified with the parameter MM (very modified).
Figure 1. Aspect of the individual films (a), packaging form (b) and original packaging (c).
Source: Authors themselves.
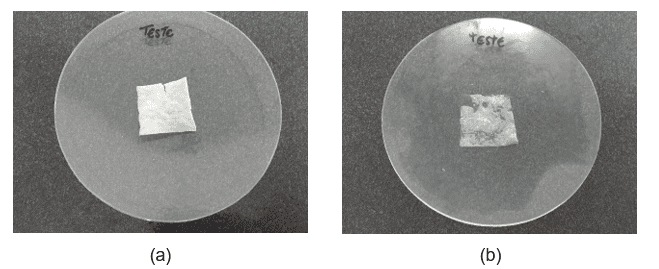
Samples stored in an oven, at a temperature of 40º ± 2ºC, underwent changes in taste, odor and appearance. The films acquired rigidity, became dry, inflexible and brittle (Figure 2), also impairing the performance of the other tests. Samples stored in a refrigerator, at a temperature of 5º ± 2ºC, also underwent noticeable changes. In this condition, the films acquired a gelatinous appearance, became sticky, inferring water absorption. Such alterations compromised the organoleptic characteristics of the samples and other physical parameters.
Figure 2. Aspect of ODF stored for seven days (T7) at 40º ± 2ºC (a) and 5º ± 2ºC (b).
Source: Authors themselves.
The results obtained demonstrate that the storage conditions and the type of packaging material are important factors to be taken into account in the development of ODF.
4. CONCLUSION
In view of the tests performed and the experimental conditions used in this study, it was possible to produce ODF using essential oils of Sicilian lemon and tangerine. The proposed formulas and the method of preparation proved to be satisfactory, the samples obtained kept their characteristics unchanged for a period of 28 hours at room temperature, however, they underwent changes when stored in an oven and refrigerator. The films produced had a very pleasant sweet taste, odor and flavor, reminiscent of Sicilian lemon (F1) and tangerine (F2). The ODF showed uniformity and good appearance, reduced disintegration time and favorable organoleptic characteristics. Although the samples suggested in this study are promising alternatives for drug delivery and release of active principles in the oral cavity, further studies must be conducted to ensure their large-scale production.
5. REFERENCES
ARYA, A. et al. Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form. Int J ChemTech Res., v. 2, n. 1, p. 576-583, 2010.
BALA, R.; KHANNA, S.; PAWAR, P.; ARORA, S. Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig, v. 3, n. 2, p. 67, 2013.
BIZZO, H. R.; MARIA, A. C. H.; REZENDE, C. M. Oleos essenciais no Brasil: aspectos gerais, desenvolvimento e perspectivas. Quimica Nova, v. 32, n. 3, p. 588–594, 2009.
BRASIL. Agência Nacional de Vigilância Sanitária. Farmacopeia Brasileira, vol. 1, Brasília: Anvisa, 2019. 546p.
COUTO, R.O. Desenvolvimento de filmes mucoadesivos para liberação de fármacos anestésicos na cavidade bucal. 2015. Tese de Doutorado. Universidade de São Paulo.
DENG, L.; KANG, X.; LIU, Y.; FENG, F.; ZHANG, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocolloids, v. 74, p. 324-332, 2018.
FERREIRA, A.O.; BRANDÃO, M. Guia prático da farmácia magistral. Pharmabooks, 2008.
FERREIRA, L.M. et al. Pullulan: an advantageous natural polysaccharide excipient to formulate tablets of alendronate-loaded microparticles. Brazilian Journal of Pharmaceutical Sciences, v. 51, n. 1, p. 27-33, 2015.
GHOSH, T.; GHOSH, A.; PRASAD, D. A review on new generation orodispersible tablets and its future prospective. Int J Pharm Pharm Sci, v. 3, n. 1, p. 1–7, 2011.
HOFFMANN, E.M.; BREITENBACH, A.; BREITKREUTZ, J. Advances in orodispersible films for drug delivery. Expert opinion on drug delivery, v. 8, n. 3, p. 299-316, 2011.
KESHARI, A.; KUMAR SHARMA, P.; PARVEZ, N. Fast dissolving oral films: an innovative drug delivery system. Structure, v. 20, n. 70, p. 50–500, 2014.
KULKARNI, A. S. et al. Exploration of different polymers for use in the formulation of oral fast dissolving strips. J Curr Pharm Res, v. 2, n. 1, p. 33–35, 2010.
MUSAZZI, U. M. et al. Poly (methyl methacrylate) salt as lm forming material to design orodispersible lms. Eur J Pharm Sci, v. 115, p. 37-42, 2018.
NAND, P. et al. Mouth Dissolving Tablets- a Novel Drug Delivery System. Intern J Appl Biol Pharm Tech, v. 1, n. 3, 2010.
ORLU, M. et al. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug delivery, v. 24, n. 1, p. 1243-1248, 2017.
PEREIRA, J.F.; MARIM, B.M.; MALI, S. Desenvolvimento de filmes orodispersíveis biopoliméricos à base de amido, goma xantana e gelatina. Iniciação Científica Cesumar, v. 21, n. 1, p. 61-70, 2019.
PERUMAL, V. A. et al. Formulation of monolayered films with drug and polymers of opposing solubilities. International Journal of Pharmaceutics, v. 358, n. 1-2, p. 184-191, 2008.
PREIS, M.; PEIN, MI.;BREITKREUTZ, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics, v. 4, n. 4, p. 551-562, 2012.
RESTA, V.G.; MALI, S. Efeito de sacarose e glicerol como plastificantes em filmes orodispersíveis de amido e gelatina. Iniciação Científica Cesumar, v. 21, n. 1, p. 15-25, 2019.
ROWE, R. C.; SHESKEY, P.; QUINN, M. Handbook of pharmaceutical excipients. Libros Digitales-Pharmaceutical Press, 2009.
RIBEIRO, T.C. et al. Filmes orodispersíveis mucoadesivos baseados em goma gelana e goma de caju para administração via mucosa oral de insulina: avaliação estrutural. Journal of Basic and Applied Pharmaceutical Sciencies, v. 38, Supl. 1, 2017.
SANFELICE, A.M.; TRUITI, M.C. Torrado. Produtos em filme-Inovação na tecnologia de cosméticos. Acta Scientiarum. Health Sciences, v. 32, n. 1, p. 61-66, 2010.
TOMAR, A. et al. Formulation and evaluation of fast dissolving oral film of dicyclomine as potential route of buccal delivery. International Journal of Drug Development & Research, v. 4, n. 2, p. 408-417, 2012.
[1] Professor at the University of São Francisco, PhD in Drugs and Medicines; Master’s in Drugs and Medicines; Specialization in Biological Sciences; Degree in Pharmaceutical Sciences. Member of the Research Group on Environment and Sustainability.
[2] Scientific Initiation Student at Universidade São Francisco, Pharmacy Course.
[3] Scientific Initiation Student at Universidade São Francisco, Pharmacy Course.
[4] Scientific Initiation Student at Universidade São Francisco, Pharmacy Course.
Sent: October, 2019.
Approved: October, 2019.