ORIGINAL ARTICLE
BEDIN, Rafael Antonio Caldart [1], SCHULTZ, Maisa [2], BEDIN, Antonio [3]
BEDIN, Rafael Antonio Caldart. SCHULTZ, Maisa. BEDIN, Antonio. Anesthesia for rabbits submitted to experimental surgeries: Report of a series of eight anesthesias. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 06, Vol. 03, pp. 151-158. June 2020. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/health/anesthesia-for-rabbits, DOI: 10.32749/nucleodoconhecimento.com.br/health/anesthesia-for-rabbits
SUMMARY
Anesthesia for laboratory animals is a matter of biomedical concern and one of the most present dilemmas in the current bioethical debate. The use of anesthetic agents in experimental surgery aims at analgesia and restraining the animal, in order to achieve a reasonable degree of muscle relaxation and to produce sufficient analgesia. This practice requires the use of protocols for the administration of safe and efficient doses. Eight New Zealand rabbits were submitted to laparotomies demonstrating the surgical technique discipline of the local medical course. For pre-anesthetic medication, acepromazine 1 mg.kg-1 associated with ketamine 15 mg.kg-1 was used subcutaneously. Anesthesia was maintained with isoflurane and oxygen under a laryngeal mask in a Mapleson D anesthesia system and under spontaneous breathing. Hydration was performed with 10 ml.kg-1 saline every hour. A thermal mattress was used. Precordial stethoscope, pulse oximetry and clinical parameters were used for monitoring. For euthanasia, ketamine 10 mg.kg-1 associated with potassium chloride 19.1% 1 ml.kg-1 was used intravenously. The average weight of the rabbits was 2721.25 ± 275.01 grams and the duration of the anesthetic procedure was 120 ± 87 minutes. Discussion. In long-term anesthesia, such as laparotomies, the use of pre-anesthetic medication and then anesthetic induction by the combination of agents is recommended. However, anesthetic management requires monitoring to prevent insufficient or excessive doses from occurring.
Key words: Rabbits, isoflurane, general anesthesia.
INTRODUCTION
Anesthesia for laboratory animals is a subject of ethical debate and has been one of the most conflicting topics in this field of knowledge (BEDIN et.al., 2013). The use of anesthetics in experimental surgery aims to contain the animal, in order to achieve a reasonable degree of muscle relaxation and produce sufficient analgesia. This practice requires the use of protocols for the administration of safe and efficient doses (BEDIN et.al., 2013).
BIBLIOGRAPHIC REVIEW
Rabbits usually present stress due to preoperative management and during preanesthetic medication and during induction with intravenous or volatile anesthetic agents. Here you can add stress with the manipulation of the animal for the administration of anesthesia and may result in respiratory and cardiac arrest. The occurrence of respiratory infection by Pasteurella multocida can lead to severe respiratory complications during anesthesia. In the preoperative examination of these animals, we should pay close attention to the presence of respiratory symptoms (runny nose and noises adventitious to auscultation), which may be strong indications of respiratory tract infection (BEDIN et.al.,2013).
Anesthesia in rabbits can range from simple sedation to deep anesthesia planes, which allow smaller and larger surgical procedures. The use of halogenated volatile anesthetics in rabbits is often used for long-term procedures especially when it is intended to keep the homeostasis of the animals as balanced as possible (BEDIN et.al., 2013).
After administration of preanesthetic medication, it is 15 minutes to adapt the laryngeal mask, connecting it to the anesthesia device. O2 from 50 to 100% and isoflurane from 1 to 5% (BEDIN et.al.,2013; RAILLARD et.al.,2019; TUNCALI et.al., 2018). The tracheal intubation procedure presents great technical difficulty when the animals are rabbits (BEDIN et.al., 2013). Visualization of the glotwith laryngoscope is practically impossible in these animals (BEDIN et.al., 2013). The blind-oriented technique of breathing is usually the most frequent mode for intubation in rabbits. Due to the difficulty of tracheal intubation, tracheostomy or laryngeal masks are used by many who work in this area (THOMPSON et.al., 2017). Anesthetic procedures in rabbits can be kept under facial mask or laryngeal mask with oxygen and halogenated anesthetic agent in spontaneous breathing (BEDIN et.al., 2013).
In procedures in which pulmonary ventilation is needed, this can be manual, with the use of a Mapleson D system, or mechanical controlled ventilation with a circular filter (BEDIN et.al., 2013). Monitoring is primarily clinically based by observing vital data such as heart rate, respiratory rate, mucosal staining, and presence of oculopalpebraal reflexes. They are routinely used for this monitoring precordial stethoscope, electrocardioscope and pulse oximetry. The other monitors should be used according to the needs imposed by the study design, and capnographs, invasive and noninvasive blood pressure may be useful.
The postoperative period is part of the anesthetic protocol, and the care given to the animal is fundamental at this time. Complications such as respiratory depression, which can occur during the anesthetic act, become fatal in this period. After the end of the anesthetic-surgical procedure, the animals should be referred for anesthetic recovery. This place should remain silent, with low light, and to avoid the stress of the animals, one must have minimal handling. If euthanasia is provided for in the study protocol the techniques for this mister should provide unconsciousness followed by cessation of the heartbeat and respiratory and definitive termination of brain functions. Ideally, the technique would minimize the anxiety experienced by the animal before loss of consciousness.
For rabbits, the most used techniques for euthanasia are: inhaled CO2, inhaled agents, venous barbiturates and venous potassium chloride, which is only being used in animals under deep sedation because venous injection is painful (BEDIN et.al., 2013).
Based on these data from the literature, it has been adopted as an anesthetic procedure used as routinely in the discipline of Operative Technique and Anesthesiology of the Medical Course of the University of Joinville region (UNIVILLE) for anesthesia in rabbits (BEDIN et.al., 2013):
- After identification, the weighing is performed: this identification and the registration of the animals are integral parts of the preoperative period. After weighing, this animal must be identified with its number and the corresponding weight.
- For preanesthetic medication, acepromazine 1 mg.kg-1 and ketamine 15 mg.kg-1 are used around fifteen to twenty minutes before the beginning of trichotomy.
- Trichotomy is performed in the anterior region of the neck, abdomen and ear margin.
- Immobilization is performed on the operating table at all:00.
- Venous catheterization is performed in the marginal vein of the ear, with a 24 G catheter. Subsequently, intravenous installation of 0.9% 250 ml saline solution is performed.
- For anesthesia ketamine 5 to 10 mg.kg-1 in sequential doses, if necessary. Laryngeal mask number 1 is used for ventilation. In certain situations (thoracic surgeries) tracheal intubation with cannula number 2 or 3 or tracheostomy is performed.
- Monitoring is performed with a precordial stethoscope (or if possible, esophageal), pulse oximetry (where available), and electrocardiocopy (where available).
- For inhalational anesthesia used for maintenance, isoflurane consists of 1% to 3% isoflurane and oxygen two to three liters per minute under mask in Mapleson D system.
- Vital signs are noted every 5 to 10 minutes for the preparation of the anesthesia form.
- During the post-anesthetic period, data are noted from the beginning of recovery until the restoration of consciousness and physiological patterns are within normal range.
- For postoperative pain control, oral paracetamol is used between 10 and 15 mg.kg-1, three times a day, having dipyrone here as an alternative.
- If the study protocol provides for euthanasia it can be performed in a CO2 inhalation chamber with 10 l.min-1 for 10 minutes. The second choice may be potassium chloride at 19.1 % 1 ml.kg-1 venous, but with the animal sedate with ketamine 1 to 10 mg mg.kg-1 venous or subcutaneous fifteen to twenty minutes before potassium chloride (BEDIN et.al., 2013).
CASE REPORT
The anesthesias that are part of this series were authorized by the Ethics Committee on Animal Use Research of the University of Joinville Region (CEUA) number 0103/2018.
Eight New Zealand rabbits submitted to laparotomies demonstrating the discipline of operative technique of the local medical course (table 1).
Table 1. Demographics.

For preanesthetic medication, acepromazine 1 mg.kg-1 was used with ketamine 15 mg.kg-1 subcutaneously (Table 2).
Table 2. Preanesthetic and intraoperative medication.
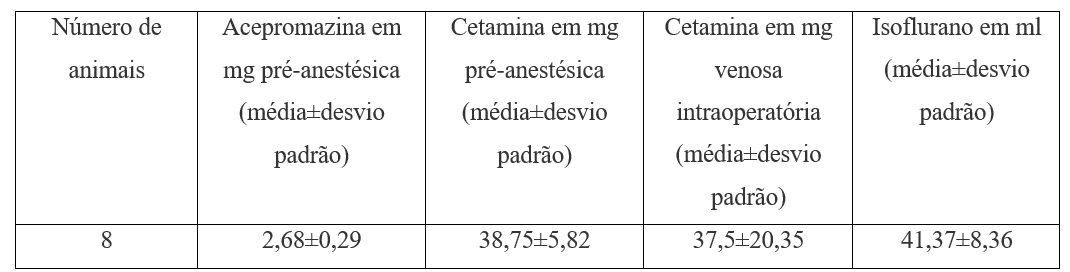
For the maintenance of anesthesia, isoflurane and oxygen was used in uncalibrated vaporizer after the passage of laryngeal mask number 1 and mapleson D inhalation anesthesia system under spontaneous breathing always. Hydration was with saline 10 ml.kg-1 (table 2). Thermal mattress was used. For monitoring, a precordial stethoscope, pulse oximetry and clinical parameters (involuntary movements, oculopalpebral reflex) were used. For euthanasia, 10 mg.kg-1 ketamine followed 5 to 15 minutes after potassium chloride 19.1% 1 ml.kg-1, also intravenously (Table 3) was used.
Table 3. Euthanasia medication.

DISCUSSION
Isoflurane is often used as a single or opioid-associated agent for anesthetic procedures in rabbits (BAILEY et.al., 2017; TEARNEY et.al.,2015). The administration of fentanyl in rabbits anesthetized with isoflurane resulted in better mean blood pressure and cardiac output, compared with isoflurane alone (BAILEY and et.al., 2017; TEARNEY et.al., 2015). Acetazine (major tranquilizer) and ketamine (dissociative anesthetic) are medications frequently used as preanesthetic and anesthetic medication for rabbits1,8,9. Both acepromazine and ketamine reduce isoflurane consumption as the main anesthetic in rabbits (BEDIN et.al., 2013; BOTMAN et.al., 2019; ECKLEY et.al., 2020).
CONCLUSION
In long-term anesthesias, such as laparotomies, it is recommended and the use of preanesthetic medication and later the anesthetic induction made by the combination of agents (ECKLEY et.al., 2020). However, anesthetic management requires monitoring to prevent insufficient or exaggerated doses from occurring. To prevent variations from happening, it is recommended to monitor vital signs from the beginning of the management of animals submitted inhalational anesthesia and the entire period during which anesthesia is maintained until euthanasia (BEDIN et.al., 2013).
REFERENCES
ATALAN, Güneri e colab. Comparison of systemic effects of midazolam, ketamine, and isoflurane anaesthesia in rabbits. Journal of Veterinary Research (Poland), v. 63, n. 2, p. 275–283, 2019.
BAILEY, Ryan S. e BARTER, Linda S. e PYPENDOP, Bruno H. Pharmacokinetics of dexmedetomidine in isoflurane-anesthetized New Zealand White rabbits. Veterinary Anaesthesia and Analgesia, v. 44, n. 4, p. 876–882, 1 Jul 2017.
BEDIN, Antonio e JUNIOR, Harry e KRELING, Patricia. Anestesia para cirurgia experimental em coelhos. v. 42, n. 2, p. 33–37, 2013.
BOTMAN, Julie e colab. Postanaesthetic effects of ketamine-midazolam and ketamine-medetomidine on gastrointestinal transit time in rabbits anaesthetised with isoflurane. Veterinary Record, 2019.
ECKLEY, Samantha S e colab. Acepromazine and Chlorpromazine as Pharmaceutical-grade Alternatives to Chlorprothixene for Pupillary Light Reflex Imaging in Mice. Journal of the American Association for Laboratory Animal Science : JAALAS, 8 Jan 2020. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/31915106>. Acesso em: 10 fev 2020.
RAILLARD, Mathieu e colab. Anaesthetic and perioperative management of 14 male new zealand white rabbits for calvarial bone surgery. Animals, v. 9, n. 11, 1 Nov 2019.
TEARNEY, Caitlin C. e BARTER, Linda S. e PYPENDOP, Bruno H. Cardiovascular effects of equipotent doses of isoflurane alone and isoflurane plus fentanyl in New Zealand white rabbits (Oryctolagus cuniculus). American Journal of Veterinary Research, v. 76, n. 7, p. 591–598, 6 Jul 2015.
THOMPSON, Krista L. e MEIER, Thomas R. e SCHOLZ, Jodi A. Endotracheal intubation of rabbits using a polypropylene guide catheter. Journal of Visualized Experiments, v. 2017, n. 129, 13 Nov 2017.
TUNCALI, Bahattin e colab. Retrospective Evaluation of Patients who Underwent Laparoscopic Bariatric Surgery. Turkish Journal of Anesthesia and Reanimation, v. 46, n. 4, p. 297–304, 16 Ago 2018. Disponível em: <http://www.ncbi.nlm.nih.gov/pubmed/30140537>. Acesso em: 29 set 2019.
[1] Graduating in medicine.
[2] Medical school graduate.
[3] Anesthesiologist. Master of Health. Doctor of Anesthesiology.
Sent: April, 2020.
Approved: June, 2020.















