ARTIGO DE REVISÃO
SANT’ANA, Danielle Carvalho [1], PEREIRA, Jéssica Petrine Castro [2], CESAR, Pedro Henrique Souza [3], TRENTO, Marcus Vinicius Cardoso [4], BRAGA, Mariana Aparecida [5], BORGES, Bruno Del Bianco [6], MARCUSSI, Silvana [7]
SANT’ANA, Danielle Carvalho. Et al. How do phenolic compounds act in the prevention and treatment of cancer? Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 07, Ed. 08, Vol. 02, pp. 77-121. August 2022. ISSN:2448-0959, Access link in: https://www.nucleodoconhecimento.com.br/health/phenolic-compounds, DOI: 10.32749/nucleodoconhecimento.com.br/health/phenolic-compounds
ABSTRACT
Cancer is the transformation of healthy cells into tumor cells in a multistage process that can affect any organ of the body. This disease is the second leading cause of death globally. There are now more than 10 million cancer cases per year worldwide, and the most common occurs in the lung, breast, and colon. Several studies demonstrate that lifestyle and eating habits are directly related to tumor cells’ appearance, such as high body mass index, low fruit and vegetable intake, lack of physical activity, and tobacco and alcohol use. It is widely believed that diet and nutrients can act as cancer risk-modifiers throughout the process of carcinogenesis, including initiation, promotion, progression, and/or conversion. Different plants have been the source of therapeutic agents. Plant-derived compounds have become indispensable for modern pharmacotherapy, and phenolic compounds are one of the most investigated due to their antitumor activity. Their cellular targets and mechanisms are compiled in the present work. Phenolic compounds are secondary metabolites present in large quantities in medicinal herbs and dietary plants (e.g., fruits, vegetables, and spices). They possess a diverse range of beneficial biological activities, which contribute to their potent effects on inhibiting carcinogenesis. Some benefits include cell proliferation and angiogenesis inhibition and anti-inflammatory, antioxidant, and antimetastatic activities. Thus, the present review supports the recommendations for consuming foods and drinks rich in phenolic compounds to prevent and possibly treat cancer.
Keywords: Anticancer action, Cancer prevention, Secondary metabolites, Medicinal plants.
1. INTRODUCTION
Cancer is a growing health problem worldwide and is the second leading cause of death after heart disease. According to a recent report by the World Health Organization (WHO), from 18 million cancer cases worldwide in 2018, almost 50% resulted in deaths. An estimate suggests that there will be more than 23 million new cases of cancer and more than 13 million cancer-related deaths worldwide by 2030 (INTERNATIONAL AGENCY FOR RESEARCH ON CANCER, 2020). This steady increase in case numbers highlights the urgency of increasing the efforts to promote cancer-prevention initiatives.
Cancer is a generic term to describe a set of diseases marked by an autonomous and rapid expansion of a somatic clone. Carcinogenesis (i.e., cancerous tissue development) is a complex phenomenon, which can be classified into three major steps: initiation, promotion, and progression. Tumor initiation is a quick process related to exposure to carcinogenic agents (how they are distributed into the cells and their interaction with DNA), which results in genotoxic effects. Cancer promotion is a prolonged and reversible stage related to the proliferation of cancerous cells. The last stage is progression, which corresponds to tumor cells’ invasion, growth, and metastasis (STEWARD and BROWN, 2013). Cancerous cells activate multiple cellular pathways that allow them to modify the microenvironment in its favor and overcome cellular limitations of proliferation, development, and immune surveillance (HANAHAN and WEINBERG, 2011).
The transformation of healthy cells into tumor cells arises from a combination of environmental and genetic factors. However, it is widely known that prolonged use of tobacco, alcohol abuse, some infectious diseases, poor dietary habits, and a sedentary lifestyle are risk factors (WU et al., 2018).
The association between nutrition and health promotion is a broad and challenging subject. Though there is a sharp increase in adopting healthy habits, driven mainly by social media, poor dietary habits are still considered a significant risk factor for cancer development. Evidence relating nutrition to cancer is shown in several works, including animal, analytical epidemiology, and population behavior studies (MAYNE et al., 2016). Bioactive compounds present in food have affected critical aspects of cell growth, development, and metabolic processes. In addition, food nutrients may act alone or in combination, promoting carcinogenesis inhibition and adequate cellular processes such as apoptosis and differentiation (CHAN et al., 2018).
Medicinal plants are the subject of intense research for their diverse and biologically active secondary metabolites and derivate compounds. Phytochemicals bearing an aromatic ring and one or more hydroxyl groups are called phenolic compounds, to which more than 8,000 compounds have been identified (TUNGMUNNITHUM et al., 2018). When compared to conventional chemotherapy, many of these phytochemicals have yielded promising results in cancer therapy while reducing adverse effects. Therefore, medicinal plants have become an indispensable tool for the pharmaceutical industry and cancer treatment.
This review will discuss the importance of phenolic compounds in cancer treatment and prevention, highlighting their mechanism of action.
2. CONVENTIONAL CANCER TREATMENTS
Conventional cancer treatments involve surgery, radiation therapy, medications, and other interventions to remove, shrink, and stop cancer progression. They are divided into primary (focuses on removing cancer by surgery, radiotherapy, or chemotherapy), adjuvant (kill any cancer cells that may have remained), and palliative (help attenuate the adverse effects of the previous treatment steps) treatments.
Antitumoral drugs used in chemotherapy are cytotoxic and can be used alone or in combination. The cytotoxic agents rapidly kill dividing cells by binding to mitosis components and/or DNA replication machinery. The second class of drugs targets specific pathways that are essential for cancer survival and spreading. The most common mechanisms of action are alkylating agents, topoisomerase inhibitors, tubulin binding agents, and antimetabolites (SINGH et al., 2016). Alkylating agents are compounds that transfer an alkyl group to a guanine base of DNA, preventing the double helix’s strands from correctly binding, causing cross-linking and ruptures. Topoisomerase inhibitors stop DNA replication, RNA transcription, and repair of DNA damage. Tubulin binding agents affect cell mobility and division. These compounds inhibit cell mitosis by binding to tubulin during mitotic spindle formation and prevent the polymerization and depolymerization in the microtubules. The antimetabolites chemotherapy agents act as purine and pyrimidine analogs that affect DNA replication, resulting in cell death.
Although these chemotherapy agents have been used with great success, they induce several adverse effects (HAUNER et al., 2017). The major problem is the lack of discrimination between healthy and cancer cells. Several cells in our body proliferate quickly and have metabolic rates similar to tumor cells. Chemotherapy medications target cells that divide rapidly and have intense metabolisms, such as bone marrow, heart, hair follicle, and digestive tract cells. Consequently, patients under these medications deal with cardiac problems, immunosuppression, chronic inflammation, and hair loss.
The high dosages needed to affect cancer cells aggravate the adverse effects and induce resistance to available drugs, reducing the rate of success (MANSOORI et al., 2017). Therefore, chemotherapy researchers are trending to develop more cancer-specific medications with fewer adverse effects. In this sense, bioactive compounds present in plants are a good source of new compounds with such characteristics. The development of new medicines extracted from natural compounds represents one of the most promising fields of the pharmaceutical industry. There has been an increasing thrust for identifying and isolating natural molecules that exhibit inhibitory properties towards tumor growth and metastasis (CHOUDHARI et al., 2020). These natural products have been vital for the development of multi-agent treatment regimens currently employed in cancer chemotherapy, which are used in the treatment of a variety of malignancies.
3. PHENOLIC COMPOUNDS AND CANCER
Phenolic compounds are secondary metabolites that have an aromatic ring bearing one or more hydroxyl groups. They have been subdivided into phenol carboxylic acids, flavonoids, tannins, coumarins, lignans, quinones, stilbenes, curcuminoids, chalcones, and alkaloids (GAN et al., 2019). Phenolic compounds are especially found in medicinal plants, which account for a significant part of their pharmacological properties. More than 8,000 phenolic compounds have already been reported (COSTEA et al., 2018).
Prior researches have demonstrated that the beneficial effects of dietary phenols include antioxidant, anti-inflammatory, and anticlastogenic activities (VELU et al., 2018). Several studies have tested the ability of crude extracts rich in phenolic compounds or fractions containing a mixture them to inhibit cancer progression and development, both in vitro and in vivo (KUETE et al., 2016, MULLER et al., 2019; RAHAIEE et al., 2020). Given the great structural diversity of bioactive plant-derived compounds, defining structure-activity relationships is difficult to deduce their underlying molecular mechanisms (KAPINOVA et al., 2018).
However, the anti-carcinogenic effects of phenolic compounds are primarily due to the ability to induce cell cycle arrest, inhibit oncogenic signaling cascades of cell proliferation, angiogenesis, and stimulation of apoptosis (Figure 1). Besides that, phenolic compounds also modulate ROS levels and pro- and anti-inflammatory cascades, promote transcription of tumor suppressor proteins such as p53, and help cell differentiation and correct development (SINGH et al., 2016; CHOUDHARI et al., 2020).
Figure 1. Main steps at which polyphenols may treat cancer.
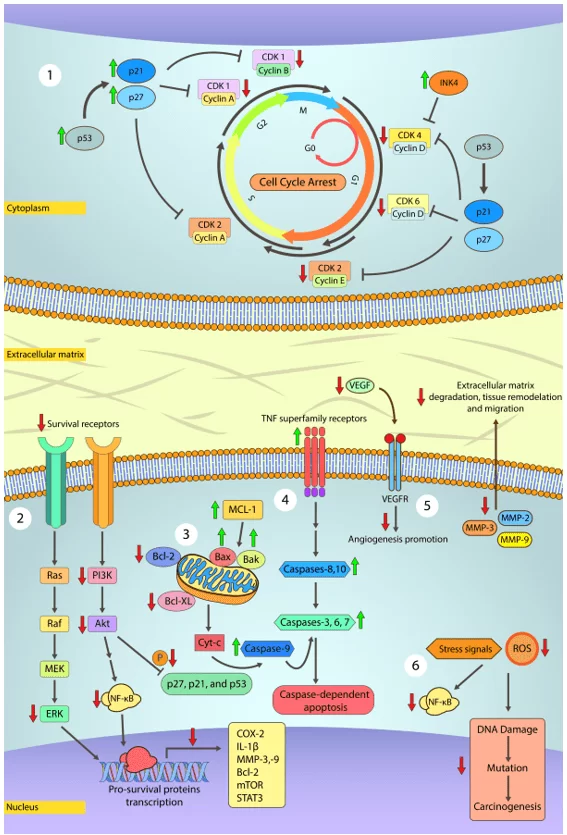
2) MAPK and PI3K/Akt pathways are both related to cancer cell proliferation, survival, differentiation, and motility. In general, MAPK and PI3K/Akt pathways are constitutively activated in human cancer and lead to the activation of pro-survival genes, namely COX-2, IL-1b, MMP-2,3,9 (cleave of extracellular matrix components, growth factors, and cell adhesion proteins; allow migration, invasion, metastasis, and angiogenesis), Bcl-2 (antiapoptotic protein) and mTOR (regulates protein synthesis, cell growth, and proliferation). Inactivation of Akt pathway prevents the phosphorylation of p27, p21, and p53 proteins, previously described as cell cycle controllers. Prior studies have shown that some polyphenols like Gambogic acid and Epigallocatechin gallate arrest MAPK and PI3K/Akt downstream signaling.
3) Apoptosis is a tightly controlled process in most cells and is critically important for life and maintaining homeostasis. Conversely, cancer cells fail to undergo programmed cell death. Polyphenols may promote apoptosis in cancer cells by inducing the release of cytochrome c from mitochondria, activation of caspases-3, -8, and -9 while downregulating Bcl-2, Bcl-XL expression via modulation of NF-κB signaling.
4) TRAIL is a death ligand from the TNF superfamily and is implied in transducing apoptosis signals in cancer cells without harming normal cells. Alongside TRAIL, other members of this superfamily, e.g., FasL/FasR, TNF-a/TNFR1, and Apo3/DR3, are the best-characterized ligands. Polyphenols may act upon such ligands and increase their sensitivity and transcription via p53 downstream signals. These ligands, upon activation, recruit cytoplasmic adapter proteins that signal and initiate the intrinsic apoptotic pathway by activating caspase-8, which in turn activate caspase-3 to initiate cell degradation. Caspase-8 also promotes the release of cytochrome c in the intrinsic pathway.
5) Angiogenesis is a key process characterized by sprouting novel blood vessels that allow cancer cells to grow rapidly and metastasize. VEGF is the main regulator of angiogenesis and is upregulated by NF-κB. Several polyphenols like luteolin and resveratrol have been shown to reduce VEGF expression and secretion.
6) At high concentrations, free radicals pose as a risk for cell functionality. Free radicals damage cell structures and biomolecules, especially DNA, which can result in the development of cancer and many other health issues. Due to the intense metabolism in cancer cells, free radicals production occurs at a higher rate. In general, polyphenols are able to scavenge and neutralize free radicals avoiding further damage to neighboring cells.
Source: Own authorship (2020).
Resveratrol, a phytoalexin able to trigger anti-proliferative and pro-apoptotic mechanisms, serves as an excellent example of the impacts of administering phenolic compounds to treat diseases. The main actions are modulation of pro-oncogenic miRNAs and tumor suppression; NFkB, PPAR, PGC1α, NRF1,2, p53 transcription factors, and TGFβ signaling targeting pathways (WRIGHT et al., 2017; FERRAZ DA COSTA et al., 2018; SUN et al., 2019). In a computational study, resveratrol and some derivatives targeted the αvβ3 integrin receptor, which could result in a blockade of cell proliferation and migration (HO et al., 2018). Subbaramaiah and coworkers showed that the mixture of several dietary polyphenols from Zyflamend®, including resveratrol, EGCG, and curcumin, suppressed levels of pro-inflammatory mediators (TNF-α, IL-1β, COX-2, phospho-Akt, phospho-p65, NF-κB-binding activity) in the mouse model of obesity-associated mammary gland inflammation (SUBBARAMAIAH et al., 2013). Acacia hydaspica R. Parker is known to possess several medicinal properties. Four active polyphenols, namely, 7-O-galloyl catechin, catechin, methyl gallate, and catechin-3-O-gallate, have been linked to inhibit cell growth and survival in prostate cancer (PC-3) and breast cancer (MDA-MB-231) cells. The polyphenols induced apoptosis by targeting various signaling factors such as PI3-K, JAK2, Akt-1, STAT3, MAPK (ERK1/2, pERK1/2), NFκB, Bcl-xL and survivin. Further, those compounds inhibited NFκB pathway activation, which are related to initiation and acceleration of tumorigenesis, suggesting its chemopreventive role (HOFFMANN and BALTIMORE, 2006; AFSAR et al., 2016).
In some experimental studies, phenolic compounds acted upon angiogenesis, an important landmark for cancer progression and migration. Mojzis and coworkers used the Flavin7, a flavonoid complex supplement, in an in vitro testing and reported its antiangiogenic activity in HUVEC-lines (MOJZIS et al., 2008). In another study, conducted by Huang et al. (2020) using the cell lineage SK-BR-3 of breast cancer, oregano flavonoids showed the ability to inhibit the growth of mammary tumor cells by suppressing of the VEGF/VEGFR-2, MMP3 and MMP9 signaling pathways (HUANG et al., 2020).
The study from Martino et al. (2019) used a polyphenol rich extract from Annurca apple (APE) upon breast cancer MDA-MB-231 cell. Researchers treated the cells with APE and analyzed cell cycle progression by flow cytometry. Cellular population in phase G2/M increased significantly in treated cells by a p21-independent process. This is particularly important because p21 behaves like a dual regulator. Depending on the cellular type, p21 may suppress or promote tumor development. APE, like other polyphenols extract, acts upon a multitude of tumor-related pathways. In the same study, APE shown to inhibit ROS/NF-kB anti apoptotic pathway and activated ROS/JNK pro-apoptotic pathway (MARTINO et al., (2019). More mechanisms of action are described in the table bellow (Table 1).
Table 1. Representative compounds within the different subclasses of phenolic compounds and their respective molecular targets in the human organism.
| Subclass | Compounds | Molecular targets |
| Hydroxybenzoic acids | 3,4-Dihydroxybenzoinc acid | Apoptosis mediated by Fas/FasL pathway (LIN et al., 2007).
Upregulation of pro-apoptotic mediators (LIN et al., 2007).
|
| Ellagic acid | Stimulate expression of caspases-3 and -9 and Bax (LIU et al., 2017).
Reduce the expression of pro-inflammatory markers via NF-kB (LIU et al., 2017).
|
|
| Inhibition of metastasis (LIU et al., 2017). | ||
| Reduce the expression of PI3K, metastasis and invasiveness (WANG et al., 2019). | ||
| Hydroxycinnamic acids | Caffeic acid | Cell cycle arrest at G0/G1 phase and increase in caspases-1, -3 and -8, expression resulting in apoptosis (PELINSON et al., 2019). |
| Sinapic acid | Reduction in MMPs expression preventing metastasis, increase in apoptosis related effectors (Eroğlu et al., 2018). | |
| Coumarins | Fraxin
Auraptene Warfarin Calanolide A Novobiocin |
Cell cycle arrest (MENEZES and DIEDERICH, 2019; MOHAMED et al., 2019;
Activation of caspases-3 and -9 (ELSHEMY and ZAKI, 2017). Antiproliferative effect (HAO et al., 2019; GARG et al., 2020) Inhibition of NF-kB (YIN and YU, 2018; HASSANEIN et al., 2020). |
| Xanthones | Mangostin
Desoxygartanin Garcinone
|
Downregulation of Cyclins/CDKs expression (LIU et al., 2019; NAUMAN et al., 2020). |
| 1,5,8-trihydroxy-3-methoxy xanthone | Inhibition of angiogenesis and metastasis by reducing the expression of VEGF and MMP-2 and -9 (BARUA et al., 2020; KLEIN-JÚNIOR et al., 2020). Inhibition of PI3k/Akt and ERK-dependent pathways (BARUA et al., 2020). | |
| Chalcones | Flavokawain B
Lonchocarpin Chalcone-Indole derivatives Chalcone-imide derivatives Chalcone-quinoline derivatives |
Inhibition of CDKs and cell cycle arrest (BORTOLOTTO et al., 2017).
Activation of Caspase pathway (BORTOLOTTO et al., 2017). Upregulation of pro-apoptotic genes (BORTOLOTTO et al., 2017).
|
| Stilbenoids | Resveratrol
|
Cell cycle arrest via activation of p21 and p27 (HEO et al., 2018).
Reducing the expression of the oncogenic microRNA, miR-221 NF-κB (WU and CUI, 2017). |
| Piceatannol | Reduce the expression of p38, MAPK and c Myc; and cleavage of caspase-3 (LUCAS et al., 2018; BANIK et al., 2020). | |
| Lignans | Sesamin
|
Increase the expressions of Bax and caspase-3; and inhibition of mTOR leading to autophagy (DEESRISAK et al., 2020). |
| Honokiol | Inducing the release of mitochondrial cytochrome c to cytosol
Regulation of EGFR signaling pathway (XIE et al., 2016; YANG et al., 2017) |
|
| Lignins | – | |
| Flavonoids | ||
| Flavones | Apigenin | Cytotoxic effect via direct targeting of mitochondria, leading to increased ROS production and cytochrome c releasing (SALMANI et al., 2017; WANG and
ZHAO, 2017) |
| Chrysin | Induction of Endoplasmic reticulum stress, inhibition of PI3k/AKT pathway and apoptosis mediated by activation of ERK1/2 MAPK and p38 MAPK pathways (RYU et al., 2017). | |
| Luteolin | Increase in BAX/Bcl-2 ratio and cell cycle arrest at G1/S phase (LU et al., 2017; ZHANG et al., 2017). | |
| Flavonols | Quercetin | Apoptosis and cell cycle arrest at phase G2/M induced by reactivation and increased expression of p53 (CHOU et al., 2010; CLEMENTE-SOTO et al., 2019) |
| Kaempferol
|
Promotion of cell death via the IRE1-JNK-CHOP pathway (KIM et al., 2018). | |
| Chrysosplenol d | Increased and sustained ERK1/2 activation resulting in apoptosis (LANG et al., 2020). | |
| Flavanones | Hesperidin | Cell cycle arrest via downregulation of Cyclin D1 and upregulation of p21 and p53 (KAMARAJ et al., 2019; AGGARWAL et al., 2020). |
| Eriodictyol | Induction of apoptosis mediated by inhibiting phosphorylation of PI3K, Akt, and NF-κB (LI et al., 2020). | |
| Naringin
Naringenin |
Increase the potency of chemotherapeutic agents
Inhibits cell growth via inhibition of PI3K/AKT/mTOR signaling pathway (CHENG et al., 2020; MEMARIANI et al., 2020). |
|
| Isoflavonoids | Genistein | Cancer cells growth inhibition as a result of inhibiting Akt phosphorylation (GONG et al., 2003). |
| Vestitol
Neovestitol
|
Downregulation of cancer related proteins (NANI et al., 2018). | |
| Puerarin | Cancer microenvironment modulation, allowing infiltration and activity of immune cells, and administration of chemotherapeutic agents (XU et al., 2020). | |
| Anthocyanidins | Malvidin
|
Induction of G1/S phase cell arrest and apoptosis
Activation of p38 MAPK and suppression of Akt, resulting in growth inhibition and apoptosis (LIN et al., 2020). |
| Cyanidin
|
Inhibition of NF-κB signaling molecules and activation of the protective pathway, Nrf2 (LEE et al., 2020). | |
Source: Own authorship (2020).
4. ANTICANCER PROPERTIES OF PHENOLIC COMPOUNDS
4.1 HYDROXYBENZOIC ACIDS
Hydroxybenzoic acids compose a group of seven carbon molecules with a C6-C1 skeleton, which are produced through the shikimate pathway. These compounds are universally distributed in the plant kingdom, being gallic, protocatechuic, vanillic, and syringic acids, the four most abundant compounds. Within this group of compounds, there is a sub-group, the tannins, which vary greatly from small to large molecules (CROZIER and CLIFFORD, 2006).
Tannins are a group of polymeric phenolic substances known to demonstrate promising antimicrobial activity through the inactivation of adhesins, cell envelope, enzymes, and different transport proteins (KY et al., 2015). These compounds can be divided into two groups, namely hydrolyzable and condensed tannins. Most activities of the proanthocyanidins and hydrolyzable tannins, including antioxidant and free radical scavenging capacity, largely depend on their structure; for example, an increase in anti-radical effects was observed with an increase in the degree of polymerization (SIENIAWSKA, 2015). According to recent reports, the antimicrobial activity of plant extracts rich in gallotannin is attributed to the inactivation of membrane-bound proteins (FENG et al., 2011).
More than 32 species in 112 tested traditional Chinese medicinal plants associated with anticancer contain tannin constituents, such as gallotannins (CAI et al., 2004). It was observed in a study that a gallotannin-rich extract obtained from pods and seeds of Caesalpinia spinosa induces loss of mitochondrial membrane potential (ψm), nuclei fragmentation, and caspase-dependent apoptosis in vitro in several tumor cells (CASTAÑEDA et al., 2012). Ellagitannins represent an important class of phytochemicals that are being increasingly investigated for their chemopreventive and anticancer activities (ISMAIL et al., 2016). Proanthocyanidins (GSPs) can be found in many plants, but this powerful compound is most abundant in the bark of the maritime pine and grapes. GSPs have demonstrated many biological properties in studies with grape seeds (AGUIAR et al., 2014), including antioxidant and anticancer effects (GAO et al., 2018).
The extract of the transformed root of Leonurus sibiricus was tested upon glioma cells (grades I-III). The extract rich in vanillic acid and gentisic acid, 4-hydroxybenzoic acid, 1,3-dicaffeoylquinic acid, and α-resorcylic acid were able to impair mitochondrial membrane potential and an increase in ROS production. Modulation of apoptosis-associated genes also triggered cell death. The extract induced apoptosis by reducing the expression of Bcl-2 and increasing the expression of Bax, p53, and caspases −3, −8, and −9 (SITAREK et al., 2017). Gallic Acid (GA), another phenolic acid well known to possess a wide range of biological activities, have been tested with success upon several cancer cell lines, such as TE-2 (esophageal cancer), MKN-28 (gastric cancer), MDA-MB-231 (breast cancer), CaSki (cervix cancer), and HT-29 and Colo201 (colon cancer). In research conducted by Lee et al. (2017), GA induced different effects depending on the cell type. For MCF-7 (breast cancer), GA triggered cell-cycle arrest at phase G2/M but not apoptosis (FARIED et al., 2007). However, for MDA-MB-231 cells, GA induced G1 phase arrest and apoptosis. The main mechanism involved in this action was up-regulation of p21 and p27, followed by downregulation of CDK-4 cyclin D1 and Cyclin E- CDK2 complexes (LEE et al., 2017). In the work of Sánchez-Carranza et al. (2017), gallic acid was further tested upon cancer cell lines with high incidence and mortality, namely PC3 (prostate), HeLa, Ca Ski (cervical), Hep3B, and HepG2 (hepatocellular) carcinoma cells. In this study, GA induced cell cycle arrest at phases S and G2/M and increased the expression of PARP, Caspases-3, and -9, which, consequently, increase Bax expression (SÁNCHEZ-CARRANZA et al., 2017). Therefore, several mechanisms of action are observed for different cell lines and forms of administration.
4.2 HYDROXYCINNAMIC ACIDS
Hydroxycinnamic Acids (HCAs) are naturally occurring compounds, and their alkyl esters may possess enhanced biological activities (MARTINI et al., 2019). Structurally, hydroxycinnamic acids are hydroxy metabolites of cinnamic acid with a C6–C3 backbone. Subclasses of these acids include caffeic, caftaric, (neo)chlorogenic, cinnamic, coumaric, and ferulic acids (often linked with dietary fibers that form esters with hemicellulose), and curcumin. Dietary sources include apples and grapes (and their juices), blueberries, cereal grains and bran, cherries, cinnamon (cinnamic acid), coffee, ginger, lettuce, olives, oranges, pears, pineapples, plums, potatoes, prunes, spinach, strawberries, sunflower seeds, turmeric, and select herbs (e.g., basal, marjoram, oregano, rosemary, sage, and thyme) (COSTAIN, 2001; SELMA et al., 2009; MORENO-JIMÉNEZ et al., 2019).
Caffeic acid (3,4-dihydroxycinnamic acid) is a phenolic compound widely distributed in medicinal plants, including fruits, vegetables, wine, coffee, olive oil, and others. Therefore, it is present in human plasma in a diet-dependent concentration (NARDINI et al., 1998; MILES et al., 2005). Based on the antioxidant and antiangiogenic effects of caffeic acid, retinal revascularization of Retinopathy Of Prematurity (ROP) could be a target for the pharmacological application of caffeic acid (KU et al., 2016). Furthermore, caffeic acid has been shown to possess anti-inflammatory and protective effects against nickel-induced oxidative liver damage (NARDINI et al., 2000; PARI and RASATH, 2008). Many studies have indicated that the reactive oxygen species scavenging and anti-inflammatory activities observed in herbs may be attributed to the various natural phenolic components (e.g., chlorogenic acid, caffeic acid, isoquercitrin, and quercetin) that have antioxidant effects (BOSE et al., 2009; DAMASCENO et al., 2017). In this context, these natural compounds’ anticancer potential is confirmed since inflammatory processes and reactive species activity are closely associated with the origin and maintenance of various types of cancer (XU et al., 2017).
Curcumin is a trending flavonoid described as chemosensitizing and that possesses a modulating role on signaling pathways involved in apoptosis, cell proliferation, tissue invasion, metastasis, and angiogenesis (MORTEZAEE et al., 2019). The therapeutic benefits of curcumin have been demonstrated in multiple chronic diseases, such as inflammation, arthritis, metabolic syndrome, liver disease, obesity, neurodegenerative diseases, and, above all, in several cancers (GIORDANO and TOMMONARO, 2019). Curcumin anticancer properties arise from downregulating signaling pathways essential to cancer development and progression, which includes NF-κB, STAT3, Activated Protein-1 (AP-1), Epidermal Growth Response-1 (Egr-1), cyclin D1, the antiapoptotic genes Bcl-2, Bcl-xL, Cyclooxygenase 2 (COX-2), and Matrix Metalloproteinase 9 (MMP-9) (GIORDANO and TOMMONARO, 2019). Despite its poor aqueous solubility and low bioavailability, curcumin has been the subject of many works due to its outstanding holotropic effect, being tested alone and in combination with conventional chemotherapy drugs. In addition, curcumin has been associated with nano-particulate delivery systems, improving monotherapy and yielding significant results to overcome the problems related to dosage and delivery.
An interesting case was the encapsulation of curcumin to a Glycyrrhetinic Acid (GA)-modified chitosan-cystamine-poly(є-caprolactone) copolymer (PCL-SS-CTS-GA) micelle for co-delivery of DOX and curcumin to hepatocellular carcinoma. In this work, curcumin was encapsulated in the form of a pH-sensitive drug (YAN et al., 2016). After internalization, the contact with the acidic pH inside the tumor provokes the carrier to open and release curcumin and doxorubicin. This method resulted in a higher cytotoxicity effect than the individual drugs. More in-depth information about curcumin’s nano-particulate delivery systems and its application can be found hereof (BATRA et al., 2019).
4.3 COUMARINS
Coumarins are a group of secondary metabolites characterized by a 2H-chromen-2-one-(1,2-benzopyrone, or 2H-1-benzopyran-2-one)-oxa-heterocycle, produced by many medicinal plants. This oxa-heterocyclic ring structure allows easy binding to several proteins, making coumarins and their derivatives great candidates for new drugs research (KAUR et al., 2015). It is known that coumarins are able to establish strong interactions with many protein sites such as hydrophobic and π–π stacking interactions with aromatic amino acids; binding to positively charged amino acids via cation-π interactions; hydrogen bonds; and dipole-dipole interactions (REN et al., 2018). Being the most studied pharmacological properties of coumarins are antiviral, antimutagenic, antioxidant, anti-inflammatory, and anticancer (STEFANACHI et al., 2018).
Coumarins and its derivatives are involved in different pathways in cancer, such as kinase inhibition, cell cycle arrest, angiogenesis inhibition, Heat Shock Protein (HSP90) inhibition, telomerase inhibition, antimitotic activity, carbonic anhydrase inhibition, monocarboxylate transporters inhibition, aromatase inhibition and sulfatase inhibition (GEISLER et al., 2011; SAIDU et al., 2012; LIN et al., 2014; THAKUR et al., 2015).
Current research focuses in modifying coumarins structure to yield compounds with enhanced anticancer properties. For example, coumarins substituted in position 4, originated strong antiproliferative compounds against breast carcinoma, A-549 human lung cancer, SKBr-3, MCF-7, HepG2, PLC/PRF5, and Huh7 (MORSY et al., 2015; AN et al., 2018; ZHANG et al., 2021). In addition, coumarins may be hybridized with azoles, furoxans, imines, chalcones and dimerized. The hybridization process with other pharmacophores may improve affinity and activity, reducing adverse effects and overcoming drug resistance. This process may be viewed in details in a review produced by Zhang and Xu (2019).
4.4 XANTHONES
Xanthones are secondary metabolites commonly occurring in a few higher plant families. Their high taxonomic value and their pharmacological properties, such as monoamine oxidase inhibition, anti-inflammatory, in vitro toxicity, and in vivo antitumor activity, have provoked great scientific interest (RUAN et al., 2017). Chemically, xanthones (9H-xanthen-9-ones) are heterocyclic compounds with the dibenzo-γ -pyrone framework. The xanthone nucleus is numbered according to a biosynthetic convention with carbons 1–4 being assigned to acetate-derived ring A and carbons 5–8 to the shikimate-derived ring B (FERNANDES et al., 2019).
Xanthones, similarly to coumarins, are considered privileged structures, which means that their structure allows the binding to several biological targets. Thus, these molecules have been modified and combined to other drugs to improve therapy success while reducing severe adverse effects. In literature, natural xanthones, its synthetic or chemically modified compounds have provided good results in cancer therapy.
Gambogic acid is a natural xanthone found in Garcinia maingayi and Garcinia morella trees resin. The mechanisms involved in Gambogic acid anticancer are described in several reports being the ability of altering oncogenic genes expression, activation of apoptosis cascades, inhibition of telomerase activity, and cell cycle arrest, the most described properties. Ovarian cancer is a serious condition affecting many women around the world, causing more than 125,000 deaths, and a sharp increase in the number of cases. Conventional radiotherapy and chemotherapy used to treat many types of cancers, though effective, are less selective, highly wearing and followed by severe adverse effects. Evidence from previous works have shown that Gambogic acid is a highly selective anticancer agent that acts by inhibiting NF-κB, MAPK/ERK, and PI3K/AKT signaling pathways, and have been used by Tang et al. (2017) to treat ovarian cancer. Gambogic acid arrested the cell cycle of SKOV3 cells at phase G2/M and induced apoptosis mediated by caspases-3 and -9 activation. Also, gambogic acid inhibited the binding of p65 to DNA further reducing SKOV3 cells proliferation.
Mangiferin is another natural xanthone with a wide spectrum of anticancer properties, acting upon many cancer cell lines (IMRAN et al., 2017). Mangiferin has been shown to suppress cyclin B1 and Akt phosphorylation, resulting in cell arrest at phase G2/M in SGC-7901 cells (DU et al., 2018). In another work, mangiferin reduced the expression of Matrix Metalloproteinases (MMP) -2 and -9 in ovarian cancer cells (ZENG et al., 2020). The high expression of MMPs observed in many cancer types, contributes to increase cancer invasiveness, metastasis and angiogenesis.
The relationship between form and biological activity is an ever-increasing research field. In special, chirality is considered one of the hottest topics in drug design, research and development. The study of enantiomers biological properties aids in the search for molecules with increased activity and with the capacity to overcome cancer resistance and evasion. Recently xanthones derivatives and its modified forms are extensively studied regarding enantio-selectivity (CALCATERRA and D’ACQUARICA, 2018).
Xanthonolignoids consists a group of xanthones bound to a phenylpropane core by a dioxane ring with pharmacological potential in cancer treatment. Synthetic kielcorins, a subclass of xanthonolignoids, have been evaluated in vitro upon MCF-7 (breast cancer), TK-10 (renal cancer), and UACC-62 (melanoma cell) cell lines (SOUSA et al., 2002). It was observed that the growth inhibitory effect was dose-dependent and dependent of isomerism. Another interesting case is of psorospermin, isolated from the bark and roots of the African plant Psorospermum febrifugum. Psorospermin contain two stereogenic centers with four possible stereoisomers, all of which have antitumor activity and topoisomerase-II-dependent DNA alkylation. However, the (R,R)-isomer of psorospermin had a better performance (HEALD et al., 2005). Further information regarding chiral modifications and derivatives of xanthones may be found here (FERNANDES et al., 2019).
4.5 CHALCONES
Chalcones are open-chain flavonoids with a common skeleton of 1,3-diphenylprop-2-en-1-one. Both naturally occurring and synthetic chalcones show an array of biological activities, including anti-bacterial, anti-viral, antioxidant, anti-inflammatory, immunosuppressive and anticancer activities (BUKHARI et al., 2013; VENTURELLI et al., 2016; GAONKAR and VIGNESH, 2017). Because of the simplicity of their chemical structures and their vast variety of actions, compounds with chalcone-based structures are currently receiving a great deal of attention (ELKHALIFA et al., 2019).
Several studies have demonstrated that chalcones can induce cancer cell apoptosis through effects on the extrinsic pathway (JANDIAL et al., 2014). Tang and collaborators (2010) demonstrated that the chalcone flavokawain B (FKB) led to the up-regulation of DR5/TRAILR2 (death receptor 5/TRAIL receptor 2). This was accompanied by an increase in cytotoxicity in vitro, as well as decreased tumor growth in vivo. Notably, there was a minimal toxic effect on normal prostate epithelial cells (TANG et al., 2010).
Lonchocarpin is a natural chalcone found in Pongamia pinnata (L.) Pierre, which have been shown to induce apoptosis in CEM leukaemic cell line (EL-GAMAL et al., 2017), and more recently in lung cancer cells (CHEN et al., 2017). Lonchocarpin was evaluated upon H292 cells and analyzed by 3D-QSAR to understand the mechanism underlying its apoptotic behavior. Data obtained indicates that lonchocarpin reduces the expression of Bcl-2 and upregulation of Bax, leading to activation of caspase-3. Further, lonchocarpin was shown to bind to BH3-binding groove of antiapoptotic proteins, contributing to caspase-3 activation and triggering apoptosis (CHEN et al., 2017).
4.6 STILBENES
The members of the stilbene family exhibit a C6-C2-C6 structure and derive from the same biosynthetic pathway as flavonoids. Although small, these compounds are widely distributed throughout the plant kingdom (CROZIER and CLIFFORD, 2006; FAUSTINO et al., 2018).
Combretastatins are natural cis-stilbenes that are isolated from the bark of the African willow tree (Combretum caffrum). The most prominent representative of this group of compounds, combretastatin A-4 (3’-hydroxy-3,4,4’,5-tetramethoxy-cis-stilbene; CA-4) exerts high antimitotic and antiangiogenic activities. Presently, phase III clinical trials are underway to test the anticancer effects of a phosphate prodrug of CA-4 (CA-4P, also known as fosbretabulin; trade name Zybrestat). CA-4P is used alone or in combination with traditional chemotherapeutic agents or with radiotherapy (SIEMANN et al., 2009; NAINWAL et al., 2019).
Resveratrol (RSV) is probably one of the most well-known polyphenols, found in a relatively small number of plants (31 genera), and in small quantities. RSV is a member of the stilbene family, which is characterized by two benzene rings linked via isopropyl moiety separated by a double bond (KASIOTIS et al., 2013). If the plant is attacked by pathogens or there is a high incidence of UV, RSV biosynthesis is increased. The historical paper of Pezzuto’s research team about the anticancer properties of resveratrol paved the way to the discovery of a wide array of therapeutical mechanisms. Said work reported that RSV acted upon all stages of cancer development – initiation, promotion and progression – and mediated anti-inflammatory response (JANG et al., 1997). Other works discloses many other beneficial properties such as antioxidant, improving circulatory health, antidiabetic and neuroprotector (ELSHAER et al., 2018).
Despite the poor bioavailability and water solubility, RSV is a promising chemopreventive and chemotherapeutic agent. Many researches have shown the action of RSV in modulating several apoptosis related proteins such as the upregulation of PARP, Caspases, BAX, BID, BAK; and downregulation and/or degradation of antiapoptotic proteins, MCL-1, Bcl-XL, and Bcl-2, in several cancer cell lines (SINGH et al., 2017). The cell cycle progression is further controlled by a series of signaling cascades that regulates DNA replication, division and proliferation of cells. The checkpoints G1, S and G2/M regulate DNA replication and cell fate, and are controlled by p53 as well as positive regulators (cyclin dependent kinases and cyclins) and negative regulators (p21WAF1/CIP1, p27KIP1). Resveratrol is implicated in the tumor suppressor p53 activation, leading to cell cycle arrest and apoptosis (SINGH et al., 2017). Administration of resveratrol at concentrations of 12.5 – 200 µmol/L for 48 h resulted in significant inhibition of colorectal cancer stem cell HCT 116 proliferation (YANG et al., 2015). Results shown an increased proportion of cells arrested in G0/G1 phase. Furthermore, RSV act as a chemo sensitization agent by increasing cisplatin cytotoxicity in CIS-resistant HCT-116 CRC cells (OSMAN et al., 2015), and to promote a reduction of NF-κB activation and translocation, mediated by a suppression of IκBα phosphorilation and degradation (WRIGHT et al., 2017). For more information regarding RSV anticancer mechanisms of action see here (ELSHAER et al., 2018; RAUF et al., 2018; VERVANDIER-FASSEUR and LATRUFFE, 2019).
4.7 LIGNANS
Lignans are naturally occurring plant phenols that are derived biosynthetically from phenylpropanoids, are commonly referred to as dimers, with complex skeletons and characteristic chemical functions, but some are trimers or tetramers. The widely distributed lignans are important components of food and medicine that are derived from plants, and they have been target compounds for organic synthesis and biological-function research because of many types of bonding of the C6–C3 units, and oxidation of the structures (SUGAHARA et al., 2008; GROSSO et al., 2017).
Different types of lignans have considerable attention because of their pharmacological features such as the Platelet-Activating Factor (PAF) antagonistic, estrogenic, antifungal, antihypertensive, sedative, antioxidant and antitumor activities (SILVA and ALCORN, 2019). Enterolactone (EL) is a bioactive phenolic metabolite known as a mammalian lignan derived from dietary lignans, has been reported to reduce risk, decrease mortality rate and improve overall survival particularly in breast, prostate, colon, gastric and lung cancer (MALI et al., 2019). Plants with high lignan contents have been used as folk medicine in China, Japan, and the Eastern World since ca. 1,000 years. Nowadays, their extensive use in traditional medicine makes lignans an important family of lead compounds for the development of new therapeutic agents based on structural modifications (GORDALIZA et al., 2004).
The famous tumor therapy drug podophyllotoxin (cyclolignanolide) was first identified in Podophyllum peltatum, which Native Americans used to treat warts, and also found in a traditional medicinal plant Podophyllum emodi var. chinense (EFFERTH et al., 2007). Sesamin, a lignan in sesame seeds (Sesamum indicum), possesses potent anticancer properties. The anticancer effects of sesamin have been mainly attributed to its anti-proliferative, pro-apoptotic, anti-inflammatory, antimetastatic, anti- and pro-angiogenic, and pro-autophagocytic activities (MAJDALAWIEH et al., 2017).
Jun and collaborators demonstrated that aschatin, fargesin, lirioresinol B dimethyl ether and magnolin decreased mRNA expression and osteolytic factor PTHrP secretion in MDA-MB-231 cells (JUN et al., 2014).
4.8 LIGNINS
Lignin is an important organic polymer which is abundant in cell walls of some specific cells (LIU et al., 2018), it is a complex of aromatic polymers that consist of monomeric subunits, i.e., p-hydroxyphenyl, guaiacyl, and syringyl units (BOERJAN et al., 2003; ROGERS et al., 2004). Along with cellulose and hemicellulose, lignin is the main component of secondary cell walls (ZHONG et al., 2011). It has many biological functions such as water transport, mechanical support and resistance to various stresses(LIU et al., 2018). The proper lignin deposition in specialized cell types is essential for the appropriate plant development (YOON et al., 2015).
Lignin fibers ingested adsorb carcinogen substances in the gut and reduce its resident time in the colon to the exposure time of carcinogen, and consequently the chances of developing cancerous polyps. Lignin is also known to possess ROS scavenging properties thus present an additional mode for preventing the development of cancer (LU et al., 1998). It is known that lignin has antioxidant properties, so oxidative stress can be controlled (QAZI et al., 2017). Addition of a P-gp modulator with the anticancer agent reduced the resistance to the drug and the binding of a ligand specifically directed the formulation to cancer cells and, therefore, the safety of normal cells can be restored (SIDDIQUI et al., 2018).
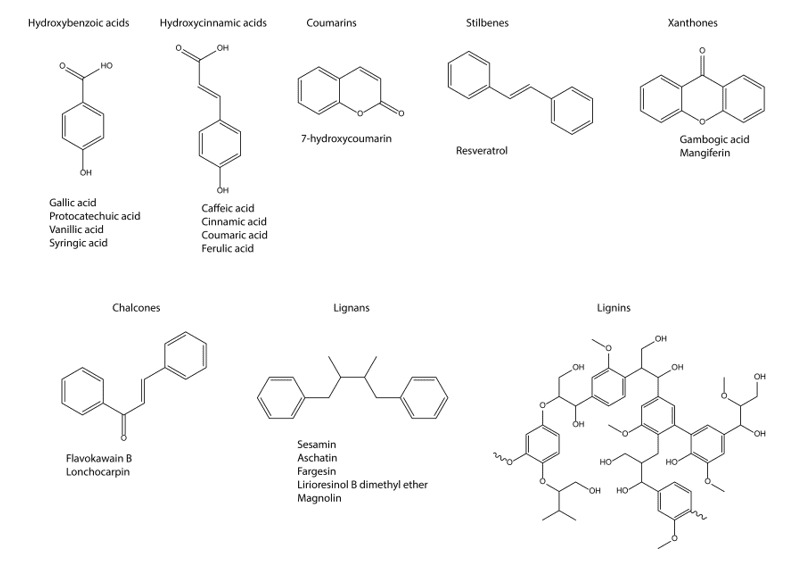
The structure of the non-flavonoid compounds may be checked on the following figure 2 (Figure 2).
Figure 2. Non-flavonoid compounds classes and some representative compounds.
4.9 FLAVONOIDS
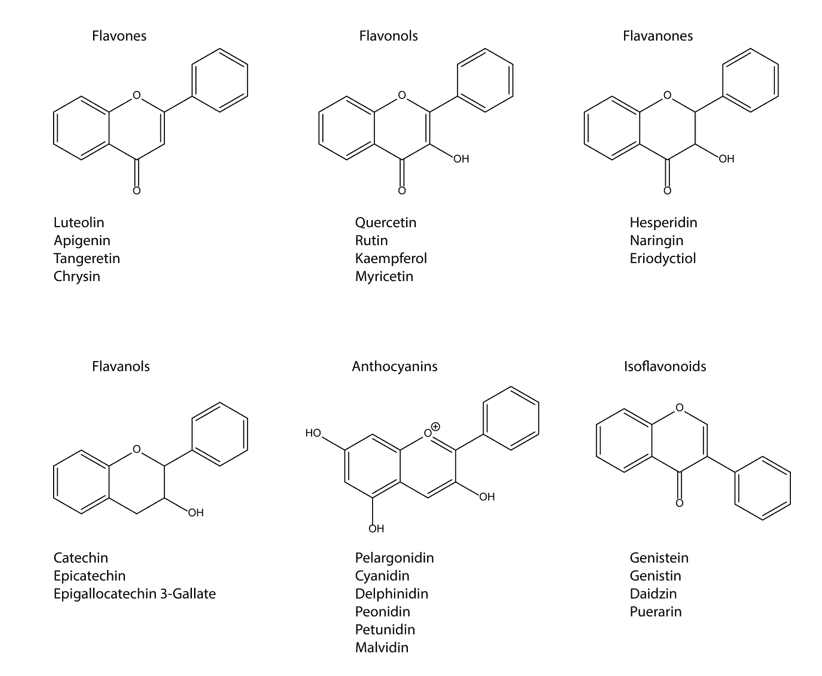
Flavonoids have been strongly linked with beneficial effects in many human, animal, and in vitro studies (MONDAL and RAHAMAN, 2020). Chemically, most flavonoids are based on a fundamental structure that has a skeleton formed by 15 carbon atoms, consisting of two benzene rings (A and B), connected through a chain, three carbons between them, and oxygen as a heteroatom (Figure 3) (SIMÕES et al., 2007).
As described by Huang et al. (2013), according to the saturation level and opening of the central pyran ring, they are categorized mainly into flavones (basic structure, B ring binds to the 2 position), flavonols (having a hydroxyl group at the 3 position), flavanones (dihydroflavones) and flavanonols (dihydroflavonols; 2–3 bond is saturated), flavanols (flavan-3-ols and flavan-3,4-diols; C-ring is 1-pyran), anthocyanins (anthocyanidins; C-ring is 1-pyran, and 1–2 and 3–4 bonds are unsaturated), isoflavonoids (mainly isoflavones; B ring binds to the 3 position), neoflavonoids (B ring binds to the 4-position), and biflavonoids (dimer of flavones, flavonols, and flavanones) (HUANG et al. 2013).
Flavonoids have been linked to reducing the risk of major chronic diseases including cancer because they have powerful antioxidant activities in vitro, being able to scavenge a wide range of reactive species. One class of compounds currently under investigation in cancer prevention are flavonoids, a large group of molecules with similar structures (CLERE et al., 2011; MAGGIONI et al., 2015). Several flavonoids have diverse bioactivities, for example, apigenin could inhibit cell adhesion and invasion; reduce the formation of diol epoxide 2, mitochondrial proton F0F1-ATPase/ATP synthase, prostaglandin synthesis, and IL-6 and 8 (interleukin) production; block the expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin; and induce cell differentiation and interferon-gamma gene expression (LINDENMEYER et al., 2001; NOEL et al., 2006). Furthermore, genistein, luteolin, quercetin, Epigallocatechin, Epigallocatechin Gallate (EGCG), and silymarin as well as apigenin show antiangiogenic and antimutagenic properties (JOHNSON, 2007).
In addition, apigenin, genistein, quercetin, EGCG, and silymarin could suppress the activation of NF-κB and AP1 and block signal transduction pathways (SURH, 2003; LINDENMEYER et al., 2001; NOEL et al., 2006; JOHNSON, 2007). Silymarin also prevented the induction of apoptosis and suppressed protein kinases and MAPKs (KIM et al., 2019). Soy isoflavone genistein was an angiogenesis inhibitor that could inhibit the growth of new blood vessels and showed antitumor and antiangiogenic activity in mouse models of melanoma and breast cancer (FARINA et al., 2006). Moreover, some isoflavones (e.g., genistein and daidzein) were phytoestrogens and could mimic the biological activity of estrogens and modulate steroid hormone metabolism. Therefore, they might play an important role in Breast Cancer (BC) prevention (MENSE et al., 2008; FAN et al., 2019).
Figure 3. Flavonoid compounds structure.
4.9.1 FLAVONOLS
Flavonols are considered the most widespread flavonoids throughout the plant kingdom, and considered, along with flavones, the major subclass of flavonoids. The most abundant compounds within the flavonols are myricetin, quercetin and kaempferol that tend to occur as O-glycosides (FERREIRA et al., 2013). Quercetin is a flavonoid widely distributed in onions, apples, red wine, tea, lemon, tomato, honey, broccoli, kale and beans (NGUYEN et al., 2017).
An antioxidant potent flavan-3-ol, EGCG, protects DNA damage and initiates repair mechanisms in various cancer models (LU et al., 2013). EGCG is the most abundant catechin in green tea and is a traditional antioxidative free radical scavenger (YIN et al., 2008). The flavanols or flavan-3-ols are the most complex subclass of flavonoids; they encompass simple molecules like catechin and polymeric proanthocyanidins. This subclass of flavonoids, unlike the others, is not planar due to a saturated C3 element in the heterocyclic C-ring (FERREIRA et al., 2013).
Catechins (tea polyphenols) namely, (-)-Epicatechingallate (ECG), (-)-Epigallocatechin Gallate (EGCG), (-)-Epicatechin (EC) and (-)-Epigallocatechin (EGC) are strong phytochemicals isolated from Camellia sinensis. Catechins have demonstrated great potential in different clinical and human trials (MAITI et al., 2017). They have strong anti-breast cancer properties by upregulating the expression of anti-oxidases such as Superoxide Dismutase (SOD), catalase and Glutathione peroxidase (GHS-px) (XIANG et al., 2016).
Molecular docking studies have confirmed that PI3K and mTOR signaling pathways are targets of Epigallocatechin Gallate (EGCG) as it strongly binds to the active site of PI3K kinase domain revealing ATP-competitive activity in MDA-MB-231 breast cancer cell line (OLIVEIRA et al., 2016). EGCG also inhibits downstream Phosphatidylinositol 3-Kinase (PI3K)/Akt activation along with EGFR signaling, down-regulates Fatty Acid Synthase (FAS) leading to growth inhibition and caspase-mediated cell death of MCF-7 BC (PAN et al., 2007). Besides, EGCG has shown an affinity to bind to target proteins like 67-kDa laminin receptor, 70 kDa Zeta-associated protein (Zap-70), Phosphoinositide 3 Kinase (PI3K), Ras-GTPase Activating Protein (GAP), vimentin and Bcl-xLand Bcl-2 to inhibit BC cells development (LI et al., 2014).
Tea catechin arrest cell cycle at G2/M phase by activating several signaling cascades, regulate cyclin A expression, phosphorylate JNK/SAPK, cyclin B1 and Cdks proteins and induces apoptosis by activating the expression caspase-3, 8, 9 enzymes (ALSHATWI, 2010; BUDISAN et al., 2017).
Catechin Gallate (CG) induces apoptosis in BC cells by down-regulating the expression of Bcl-2, Bcl-Xl followed by the suppression of PI3K, NFκB and JAK/STAT signaling cascades (AFSAR et al., 2016). A clinical trial showed that EGCG (400 mg for 2–8 weeks) has displayed promising results in human breast cancer cells by down-regulating HGF and VEGF and reducing the of MMP-2 and MMP- 9 enzymes (MAITI et al., 2017).
Quercetin has shown strong anti-BC cell effects by regulating miRNA expression. Quercetin has increased the expression of pro-apoptotic-Bax (Bcl-2 associated X) and caspase-3 and decreases the expression of oncogenic-EGFR in MDA-MB-231 and MCF-7 cells by upregulating the expression of miR-146a. Quercetin upregulates the miR-146a expression, inhibits cancer development through Bax and caspase-3 activation and inhibits invasion through down-regulating EGFR expression. Furthermore, quercetin has significantly increased miR-146a expression in xenograft cells and reduced tumor volume in mice (TAO et al., 2015). Based on these experimental studies, quercetin could be a promising anti-breast cancer agent and more detailed studies are needed (IQBAL et al., 2018).
4.9.2 FLAVONES
The flavones are structurally related to flavonols and are considered a major subclass within the flavonoids; they occur usually as 7-O-glycosides, although other substitutions can be found. The most common compounds within the flavones are apigenin, chrysin and luteolin (FERREIRA et al., 2013). Apigenin, a flavone widely found in many medicinal plants including Lycopodium clavatum L. (club moss) and some vegetables such as Petroselinum crispum L. (parsley) and Apium graveolens L. (celery), has been reported to have a beneficial effect against UV-B induced DNA damage and inflammatory diseases (SALMANI et al., 2017).
Apigenin reduces the risk of ovarian cancers as demonstrated by a population study conducted in ovarian cancer patients (GEORGE et al., 2017). Apigenin was also reported to modulate DNA damage by inhibiting casein kinase 2, a regulator of cell proliferation, the mediator of the DNA damage response and NF-κB activation in malignant glioma cells (KROONEN et al., 2012).
Luteolin, a flavone found in Salvia tomentosa Mill. (Balsamic sage) and many other plants protect SSBs induced by oxidative stress in PC12 rat pheochromocytoma cells (SILVA et al., 2008). Luteolin activates intrinsic apoptotic pathways by inducing DNA damage and p53 in many cancer cells (GEORGE et al., 2017). Chrysin reduces the disturbances of redox status, named superoxide dismutase, catalase, glutathione peroxidase and GSH in liver, kidney and brain tissues of rats treated with D-galactose (ANAND et al., 2012). In another study, chrysin protects free-radical-mediated oxidative stress induced by Nω-nitro-L-arginine methyl ester in Wistar male rats (GEORGE et al., 2017).
4.9.3 FLAVANONES
The flavanones are usually considered a minor subclass within the flavonoids due to their limited occurrence, mainly in fruits and especially in citrus (REF). Examples of flavanones are hesperidin (orange juice) and naringin (grapefruit) and due to the high levels of hesperidin in orange juice, it has a sour taste, while naringine is sweeter (FERREIRA et al., 2013). Dietary polyphenols like ellagic acid, genistein, emodin and guggulsterone have also been proved involved with the regulation of cell cycle and apoptosis in various cancer cells (LAPENNA and GIORDANO, 2009). Naringenin, present in orange peel, was found to have the potential to induce DNA damage and apoptosis in HaCaT immortalized keratinocytes and in various other cancer cells (EL-MAHDY et al., 2008). Flavanones have been reported as a potent anticancer agent, this potentially protective effect has been related to the various properties of these compounds, which include anti-oxidative and anti-inflammatory activities (MURTI and MISHRA, 2014; STEVENS et al., 2019).
4.9.4 ISOFLAVONES
Soy isoflavones, also called isoflavonoids, represent a class of chemical compounds known as phytoestrogens, linked or not to sugar molecules, and comprise aglycone: genistein (5,7,4-trihydroxyisoflavone), glycitein (7,4- dihydroxy-6-methoxyisoflavone) and daidzein (7,4-dihydroxyisoflavone). These isoflavonoids can act both as agonists, antagonists, or as modulators of selective estrogen receptors (BARNES et al., 1994). Isoflavones have one of the highest rates of absorption compared to other flavonoid classes (SELMA et al., 2009). Isoflavones have antiestrogenic as well as estrogenic effects on breast cancer cells in culture, in animal models, and clinical trials (ZIAEI and HALABY, 2017).
In vitro studies have demonstrated a weak proliferative effect of isoflavones on breast cancer cells or even blockage of these effects, which are promoted by estradiol in these cells (PITKIN, 2012). A large cohort study with over 11,000 patients revealed that consumption of soy food may be beneficial in ER-negative post-menopausal women, and then, found that soy consumption correlated with reduced incidence and mortality of breast cancer (CHI et al., 2013).
In a study of Basu and collaborators (BASU and MAIER, 2018) the analogs of isoflavones, lignans and resveratrol can modulate the expressions of cell cycle regulators, as a consequence of inhibition of the cell cycle and proliferation of breast carcinomas (BASU and MAIER, 2018).
4.9.5 ANTHOCYANIDINS
Anthocyanidins, also known as condensed tannins are present throughout the plant kingdom, especially in their glycated forms; the anthocyanins are found in fruits and flowers in a higher quantity, but also leaves, stems and roots. The most abundant anthocyanidins are pelargonidin, cyaniding, delphinidin and peonidin. Conjugation with other molecules can take place in positions 3, 5, 7, 3’ and 5’, creating bigger molecules (FERREIRA et al., 2013).
The anthocyanins and anthocyanidins have gathered the attention of the scientific community owing to their anti-inflammatory, antioxidant, and cancer-inhibitory properties. Studies have been demonstrated that anthocyanins and anthocyanidins inhibit the pro-inflammatory NF-κB pathway and suppress abnormal epithelial cell proliferation (SOUSA MORAES et al., 2019).
5. CONCLUSION
Currently, several studies emphasized, the evaluation of specific points of tumor metabolism. Immunological aspects and induction of apoptosis are being analyzed. Moreover, cell proliferation is not the only event to be fought to treat cancer, but the tumor’s ability to nourish itself, invade adjacent tissues and create new blood vessels are major obstacles for traditional therapies. Hereupon, increasing studies for new chemical treatments and alternative and/ or adjuvant therapies have been observed. The relationship between cells and components of the extracellular matrix are fundamental in the occurrence of the tumor, both for invasion and for angiogenesis mechanisms. Polyphenols have antioxidant, antitumor and anti-inflammatory properties and the consumption of plants rich in polyphenols brings health benefits, giving them a potent preventive and protective action, and strong curative potential, which has been scientifically explored in recent decades. In this way, the present review work supports the recommendations for the consumption of foods and drinks rich in phenolic compounds to the prevention and treatment of cancer.
ACKNOWLEDGEMENTS
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). The authors express their gratitude to Universidade Federal de Lavras (UFLA) for the structural and logistic support to the researchers.
REFERENCES
AFSAR, T.; TREMBLEY, J.H.; SALOMON, C.E.; RAZAK, S.; KHAN, M.R.; AHMED, K. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways. Scientific Reports, v. 6, n. 1, 23077, 2016.
AGGARWAL, V.; TULI, H.S.; THAKRAL, F.; SINGHAL, P.; AGGARWAL, D.; SRIVASTAVA, S.; PANDEY, A.; SAK, K.; VAROL, M.; KHAN, M.A.; SETHI, G. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Experimental Biology and Medicine, v. 245, n. 5, p. 486–497, 2020.
AGUIAR, T. R.; VIDAL, C. M.; PHANSALKAR, R. S.; TODOROVA, I.; NAPOLITANO, J. G.; MCALPINE, J. B.; CHEN, S. N.; PAULI, G.F.; BEDRAN-RUSSO, A. K. Dentin biomodification potential depends on polyphenol source. Journal of Dental Research, v. 93, n. 4, p. 417-422, 2014.
ALSHATWI, A. A. Catechin hydrate suppresses MCF-7 proliferation through TP53/Caspase-mediated apoptosis. Journal of Experimental & Clinical Cancer Research, v. 29, n. 1, 167, 2010.
AN, R.; HOU, Z.; LI, J.-T.; YU, H.-N.; MOU, Y.-H.; GUO, C. Design, synthesis and biological evaluation of novel 4-substituted coumarin derivatives as antitumor agents. Molecules, v. 23, n. 9, 2281, 2018.
ANAND, K.V.; JAABIR, M.S.M.; THOMAS, P.A.; GERALDINE, P. Protective role of chrysin against oxidative stress in D-galactose-induced aging in an experimental rat model. Geriatrics & Gerontology International, v. 12, n. 4, p. 741-750, 2012.
BANIK, K.; RANAWARE, A.M.; HARSHA, C.; NITESH, T.; GIRISA, S.; DESHPANDE, V.; FAN, L.; NALAWADE, S.P.; SETHI, G.; KUNNUMAKKARA, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacological Research, v. 153, 104635, 2020.
BARNES, S.; KIRK, M.; COWARD, L. Isoflavones and their conjugates in foods: extraction conditions and analysis by HPLC – mass spectrometry. Journal of Agricultural and Food Chemistry, v. 42, n. 11, p. 2466-2474, 1994.
BARUA, A.; CHOUDHURY, P.; MANDAL, S.; PANDA, C.K.; SAHA, P. Anti-metastatic potential of a novel xanthone sourced by Swertia chirata against in vivo and in vitro breast adenocarcinoma frameworks. Asian Pacific Journal of Cancer Prevention, v. 21, n. 10, p. 2865–2875, 2020.
BASU, P.; MAIER, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomedicine & Pharmacotherapy, v. 107, p. 1648–1666, 2018.
BATRA, H.; PAWAR, S.; BAHL, D. Curcumin in combination with anticancer drugs: A nanomedicine review. Pharmacological Research, v. 139, p.91-105, 2019.
BOERJAN, W.; RALPH, J.; BAUCHER, M. Lignin biosynthesis. Annual Review of Plant Biology, v. 54, p. 519-546, 2003.
BORTOLOTTO, L.F.B.; BARBOSA, F.R.; SILVA, G.; BITENCOURT, T.A.; BELEBONI, R.O.; BAEK, S.J.; MARINS, M.; FACHIN, A.L. Cytotoxicity of trans-chalcone and licochalcone A against breast cancer cells is due to apoptosis induction and cell cycle arrest. Biomedicine & Pharmacotherapy, v. 85, p. 425–433, 2017.
BOSE, J.S.; GANGAN, V.; JAIN, S.K.; MANNA, S.K. Down regulation of inflammatory responses by novel caffeic acid ester derivative by inhibiting NF-kappa B. Journal of Clinical Immunology, v. 29, n. 1, p. 90-98, 2009.
BUDISAN, L.; GULEI, D.; ZANOAGA, O.M.; IRIMIE, A.I.; SERGIU, C.; BRAICU, C.; GHERMAN, C.D.; BERINDAN-NEAGOE, I. Dietary intervention by phytochemicals and their role in modulating coding and non-coding genes in cancer. International Journal of Molecular Sciences, v. 18, n. 6, 1178, 2017.
BUKHARI S.N.A.; JANTAN I.; JASAMAI M. Anti-inflammatory trends of 1,3-diphenyl-2-propen-1-one derivatives. Mini-Reviews in Medicinal Chemistry, v. 13, n. 1, 8794, 2013.
CAI, Y.Z.; LUO, Q.; SUN, M.; CORKE, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences, v. 74, n. 17, p. 2157–2184, 2004.
CALCATERRA, A.; D’ACQUARICA, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. Journal of Pharmaceutical and Biomedical Analysis, v. 147, p. 323-340, 2018.
CASTAÑEDA, D.M.; POMBO, L.M.; URUEÑA, C.P.; HERNANDEZ, J.F.; FIORENTINO, S. A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complementary and Alternative Medicine, v. 12, 38, 2012.
CHEN, G.; ZHOU, D.; LI, X.-Z.; JIANG, Z.; TAN, C.; WEI, X.-Y.; LING, J.; JING, J.; LIU, F.; LI, N. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Scientific Reports, v. 7, n. 1, 10729, 2017.
CHI, F.; WU, R.; ZENG, Y.C.; XING, R.; LIU, Y.; XU, Z.G. Post-diagnosis soy food intake and breast cancer survival: a meta-analysis of cohort studies. Asian Pacific Journal of Cancer Prevention, v. 14, n. 4, 24072412, 2013.
CHOU, C.-C.; YANG, J.-S.; LU, H.-F.; IP, S.-W.; LO, C.; WU, C.-C.; LIN, J.-P.; TANG, N.-Y.; CHUNG, J.-G.; CHOU, M.-J.; TENG, Y.-H.; CHEN, D.-R. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Archives of Pharmacal Research, v. 33, n. 8, p. 1181–1191, 2010.
CHOUDHARI, A. S.; MANDAVE, P. C.; DESHPANDE, M.; RANJEKAR, P.; PRAKASH, O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Frontiers in Pharmacology, v. 20, p. 1614, 2020.
CHAN, M. M.; CHEN, R.; FONG, D. Targeting cancer stem cells with dietary phytochemical – Repositioned drug combinations. Cancer Letters, v. 433, p. 53-64, 2018.
CHENG, H.; JIANG, X.; ZHANG, Q.; MA, J.; CHENG, R.; YONG, H.; SHI, H.; ZHOU, X.; GE, L.; GAO, G. Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Experimental and Therapeutic Medicine, v. 19, n. 6, p. 3798–3804, 2020.
CLEMENTE-SOTO, A.; SALAS-VIDAL, E.; MILAN-PACHECO, C.; SÁNCHEZ-CARRANZA, J.; PERALTA-ZARAGOZA, O.; GONZÁLEZ-MAYA, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression‑independent manner in HPV‑positive human cervical cancer‑derived cells. Molecular Medicine Reports, v. 19, n. 3, p. 2097–2106, 2019.
CLERE, N.; FAURE, S.; MARTINEZ, M.C.; ANDRIANTSITOHAINA, R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovascular & Hematological Agents in Medicinal Chemistry, v. 9, n. 2, p. 62-77, 2011.
COSTAIN, L. Super Nutrients Handbook. Dorling Kindersley Books: New York, 2001.
COSTEA, T.; HUDIȚĂ, A.; CIOLAC, O. A.; GĂLĂȚEANU, B.; GINGHINĂ, O.; COSTACHE, M.; GANEA, C.; MOCANU, M. M. Chemoprevention of colorectal cancer by dietary compounds. International Journal of Molecular Sciences, v. 19, n. 12, p. 3787, 2018.
CROZIER, A.; CLIFFORD, M.N.; ASHIHARA, H. Plant secondary metabolites: occurrence, structure and role in the human diet. Blackwell Publishing: Oxford, 2006.
DAMASCENO, S.S.; DANTAS, B.B.; RIBEIRO-FILHO, J.; ARAÚJO D.A.M.; DA COSTA, J.G.M. Chemical properties of caffeic and ferulic acids in biological system: Implications in cancer therapy. A review. Current Pharmaceutical Design, v. 23, n. 20, p. 3015-3023, 2017.
DEESRISAK, K.; CHATUPHEERAPHAT, C.; ROYTRAKUL, S.; ANURATHAPAN, U.; TANYONG, D. Autophagy and apoptosis induction by sesamin in MOLT-4 and NB4 leukemia cells. Oncology Letters, v. 21, n. 1, 32, 2020.
DU, M.; WEN, G.; JIN, J.; CHEN, Y.; CAO, J.; XU, A. Mangiferin prevents the growth of gastric carcinoma by blocking the PI3K-Akt signalling pathway. Anticancer Drugs, v. 29, n. 2, p. 167-175, 2018.
EFFERTH, T.; LI, P.C.H.; KONKIMALLA, V.S.B.; KAINA, B. From traditional Chinese medicine to rational cancer therapy. Trends in Molecular Medicine, v. 13, n. 8, p. 353-361, 2007.
EL-GAMAL, A.A.; AL-MASSARANI, S.M.; ABDEL-MAGEED, W.M.; EL-SHAIBANY, A.; AL-MAHBASHI, H.M.; BASUDAN, O.A.; BADRIA, F.A.; AL-SAID, M.S.; ABDEL-KADER, M.S. Prenylated flavonoids from Commiphora opobalsamum stem bark. Phytochem, 141, p. 80-85, 2017.
ELKHALIFA, D.; ALALI, F.; AL MOUSTAFA, A.-E.; KHALIL, A. Targeting triple negative breast cancer heterogeneity with chalcones: a molecular insight. Journal of Drug Targeting, v. 27, n. 8, p. 830-838, 2019.
EL-MAHDY, M. A.; ZHU, Q.; WANG, Q. E.; WANI, G.; PATNAIK, S.; ZHAO, Q.; ARAFA, E.-S.; BARAKAT, B.; MIR, S. N.; WANI, A. A. Naringenin protects HaCaT human keratinocytes against UVB-induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochemistry and Photobiology, v. 84, n. 2, p. 307-316, 2008.
ELSHAER, M.; CHEN, Y.; WANG, X. J.; TANG, X. Resveratrol: An overview of its anticancer mechanisms. Life Sciences, v. 207, p. 340-349, 2018.
ELSHEMY, H.A.H.; ZAKI, M.A. Design and synthesis of new coumarin hybrids and insight into their mode of anti-proliferative action. Bioorganic & Medicinal Chemistry, v. 25, n. 3, p. 1066-1075, 2017.
EROĞLU, C.; AVCI, E.; VURAL, H.; KURAR, E. Anticancer mechanism of sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene, v. 671, p. 127-134, 2018.
FAN, X.; BAI, J.; ZHAO, S.; HU, M.; SUN, Y.; WANG, B.; JI, M.; JIN, J.; WANG, X.; HU, J.; LI, Y. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicology in Vitro, v. 61, 104642, 2019.
FARIED, A.; KURNIA, D.; FARIED, L.; USMAN, N.; MIYAZAKI, T.; KATO, H.; KUWANO, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. International Journal of Oncology, v. 30, n. 3, p. 605-613, 2007.
FARINA, H.G.; POMIES, M.; ALONSO, D.F.; GOMEZ, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncology Reports, v. 16, n. 4, p. 885-891, 2006.
FAUSTINO, C.; FRANCISCO, A.P.; ISCA, V.M.S.; DUARTE, N. Cytotoxic stilbenes and derivatives as promising antimitotic leads for cancer therapy. Current Pharmaceutical Design, v. 24, n. 36, p. 4270-4311, 2018.
FENG, Y.; WANG, N.; ZHU, M.; FENG, Y.; LI, H.; TSAO, S. Recent progress on anticancer candidates in patents of herbal medicinal products. Recent Patents on Food, Nutrition & Agriculture, v. 3, n. 1, p. 30–48, 2011.
FERNANDES, C.; CARRARO, M.; RIBEIRO, J.; ARAÚJO, J.; TIRITAN, M.; PINTO, M. Synthetic chiral derivatives of xanthones: biological activities and enantioselectivity studies. Molecules, v. 24, n. 4, 791, 2019.
FERRAZ DA COSTA, D. C.; CAMPOS, N. P. C.; SANTOS, R. A.; GUEDES-DA-SILVA, F. H.; MARTINS-DINIS, M. M. D. C.; ZANPHORLIN, L.; RAMOS, C.; RANGEL, L. P.; SILVA, J. L. Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget, v. 9, n. 49, p. 29112–29122, 2018.
FERREIRA, L.C.; GRABE-GUIMARÃES, A.; DE PAULA, C.A.; MICHEL, M.C.; GUIMARÃES, R.G.; REZENDE, S.A.; DE SOUZA FILHO, J.D.; SAÚDE-GUIMARÃES, D.A. Anti-inflammatory and antinociceptive activities of Campomanesia adamantium. Journal of Ethnopharmacology, v. 145, n. 1, p. 100-108, 2013.
GAN, R.Y.; CHAN, C.L.; YANG, Q.Q.; LI, H.B.; ZHANG, D.; GE, Y.Y.; GUNARATNE, A.; GE, J.; CORKE, H. Bioactive compounds and beneficial functions of sprouted grains. In: FENG H.; NEMZER B.; DEVRIES J.V.; editors. Sprouted Grains. AACC International Press; St. Paul, MN, USA, p. 191–246, 2019.
GAO, Y.; TOLLEFSBOL, T.O. Combinational proanthocyanidins and resveratrol synergistically inhibit human breast cancer cells and impact epigenetic mediating machinery. International Journal of Molecular Sciences, v. 19, n. 8, p. 2204, 2018.
GAONKAR, S.L.; VIGNESH, U.N. Synthesis and pharmacological properties of chalcones: a review. Research on Chemical Intermediates, v. 43, n. 11, p. 6043-6077, 2017.
GARG, S.S.; GUPTA, J.; SHARMA, S.; SAHU, D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. European Journal of Pharmaceutical Sciences, v. 152, 105424, 2020.
GEISLER, J.; SASANO, H.; CHEN, S.; PUROHIT, A. Steroid sulfatase inhibitors: Promising new tools for breast cancer therapy? The Journal of Steroid Biochemistry and Molecular Biology, v. 125, n. 1-2, p. 39-45, 2011.
GEORGE, V. C.; DELLAIRE, G.; RUPASINGHE, H. P. V. Plant flavonoids in cancer chemoprevention: role in genome stability. Journal of Nutritional Biochemistry, v. 45, p. 1-14, 2017.
GIORDANO, A.; TOMMONARO, G. Curcumin and Cancer. Nutrients, v. 11, n. 10, 2376, 2019.
GONG, L.; LI, Y.; NEDELJKOVIC-KUREPA, A.; SARKAR, F.H. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene, v. 22, n. 30, p. 4702–4709, 2003.
GORDALIZA, M.; GARCÍA, P.A.; DEL CORRAL, J.M.M.; CASTRO, M.A.; GÓMEZ-ZURITA, M.A. Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives. Toxicon, v. 44, n. 4, p. 441-459, 2004.
GROSSO, G.; GODOS, J.; LAMUELA-RAVENTOS, R.; RAY, S.; MICEK, A.; PAJAK, A.; SCIACCA, S.; D’ORAZIO, N.; DEL RIO, D.; GALVANO, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Molecular Nutrition & Food Research, v. 61, n. 4, p. 1-10, 2017.
HANAHAN, D.; WEINBERG, R.A. Hallmarks of Cancer: The Next Generation. Cell, v. 144, n. 5, p. 646-674, 2011.
HAO, S.-Y.; FENG, S.-L.; WANG, X.-R.; WANG, Z.; CHEN, S.-W.; HUI, L. Novel conjugates of podophyllotoxin and coumarin: Synthesis, cytotoxicities, cell cycle arrest, binding CT DNA and inhibition of Topo IIβ. Bioorganic & Medicinal Chemistry Letters, v. 29, n. 16, p. 2129–2135, 2019.
HASSANEIN, E.H.M.; SAYED, A.M.; HUSSEIN, O.E.; MAHMOUD, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. OxiMed & Cellular Longevity, v. 2020, p. 1-25, 2020.
HAUNER, K.; MAISCH, P.; RETZ, M. Side effects of chemotherapy. Urologe A, v. 56, n. 4, p. 472-479, 2017.
HEALD, R.A.; DEXHEIMER, T.S.; VANKAYALAPATI, H.; SIDDIQUI-JAIN, A.; SZABO, L.Z.; GLEASON-GUZMAN, M.C.; HURLEY, L.H. Conformationally restricted analogues of psorospermin: design, synthesis, and bioactivity of natural-product-related bisfuranoxanthones. Journal of Medicinal Chemistry, v. 48, n. 8, p. 2993-3004, 2005.
HEO, J.; KIM, S.; HWANG, K.; KANG, J.; CHOI, K. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. International Journal of Molecular Medicine, v. 42, n. 3, p. 1427–1435, 2018.
HO, Y.; SH YANG, Y.C.; CHIN, Y.-T.; CHOU, S.-Y.; CHEN, Y.-R.; SHIH, Y.-J.; WHANG-PENG, J.; CHANGOU, C.A.; LIU, H.-L.; LIN, S.-J.; TANG, H.-Y.; LIN, H.-Y.; DAVIS, P.J. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food and Chemical Toxicology, v. 120, p. 346-355, 2018.
HOFFMANN, A.; BALTIMORE, D. Circuitry of nuclear factor kappaB signaling. Immunological Reviews, v. 210, n. 1, p. 171-186, 2006.
HUANG, X.-F.; XUE, J.-Y.; JIANG, A.-Q.; ZHU, H.-L. Capsaicin and its analogues: structure-activity relationship study. Current Medicinal Chemistry, v. 20, n. 21, p. 2661-2672, 2013.
HUANG, X.; LI, J.; LI, M.; HUANG, J.; JIANG, X.; FU, H.; WU, J.; BAO, M.; WANG, S.; ZHANG, M.; GAO, G. Polyphenol-enriched extracts from Trapa acornis Husks inhibit Her2-positive SK-BR-3 breast cancer cell proliferation and in vivo tumor angiogenesis. Nutrition and Cancer, p. 1-12, 2020.
IMRAN, M.; ARSHAD, M.S.; BUTT, M.S.; KWON, J.-H.; ARSHAD, M.U.; SULTAN, M.T. Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids in Health and Disease, v. 16, n. 1, 84, 2017.
INTERNATIONAL AGENCY FOR RESEARCH ON CANCER. World Cancer Report: Cancer Research for Cancer Prevention. https://gco.iarc.fr/. (Accessed December 15, 2020).
ISMAIL, T.; CALCABRINI, C.; DIAZ, A.R.; FIMOGNARI, C.; TURRINI, E.; CATANZARO, E.; AKHTAR, S.; SESTILI, P. Ellagitannins in cancer chemoprevention and therapy. Toxins (Basel), v. 8, n. 5, 151, 2016.
IQBAL, J.; ABBASI, B.A.; BATOOL, R.; MAHMOOD, T.; ALI, B.; KHALIL, A.T.; KANWAL, S.; SHAH, S.A.; AHMAD, R. Potential phytocompounds for developing breast cancer therapeutics: Nature’s healing touch. European Journal of Pharmacology, v. 827, p. 125-148, 2018.
JANDIAL, D.D.; BLAIR, C.A.; ZHANG, S.; KRILL, L.S.; ZHANG, Y.B.; ZI, X. Molecular targeted approaches to cancer therapy and prevention using chalcones. Current Cancer Drug Targets, v. 14, n. 2, p. 181-200, 2014.
JANG, M.; CAI, L.; UDEANI, G.O.; SLOWING, K.V.; THOMAS, C.F.; BEECHER, C.W.W.; FONG, H.H.S.; FARNSWORTH, N.R.; KINGHORN, A.D.; MEHTA, R.G.; MOON, R.C.; PEZZUTO, J.M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, v. 275, n. 5297, p. 218-220, 1997.
JOHNSON, I.T. Phytochemicals and cancer. Proceedings of the Nutrition Society, v. 66, n. 2, p. 207-215, 2007.
JUN, A.Y.; KIM, H.-J.; PARK, K.-K.; SON, K.H.; LEE, D.H.; WOO, M.-H.; CHUNG, W.-Y. Tetrahydrofurofuran-type lignans inhibit breast cancer-mediated bone destruction by blocking the vicious cycle between cancer cells, osteoblasts and osteoclasts. Investigational New Drugs, v. 32, n. 1, p. 1-13, 2014.
KAMARAJ, S.; ANANDAKUMAR, P.; JAGAN, S.; RAMAKRISHNAN, G.; PERIYASAMY, P.; ASOKKUMAR, S.; SUBRAMANIAN, R.; DEVAKI, T. Hesperidin inhibits cell proliferation and induces mitochondrial-mediated apoptosis in human lung cancer cells through down regulation of β-catenin/c-myc. Biocatalysis and Agricultural Biotechnology, v. 18, 101065, 2019.
KAPINOVA, A.; KUBATKA, P.; GOLUBNITSCHAJA, O.; KELLO, M.; ZUBOR, P.; SOLAR, P.; PEC, M. Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environmental Health and Preventive Medicine, v. 23, n. 1, p. 36, 2018.
KASIOTIS, K.M.; PRATSINIS, H.; KLETSAS, D.; HAROUTOUNIAN, S.A. Resveratrol and related stilbenes: their anti-aging and antiangiogenic properties. Food and Chemical Toxicology, v. 61, p. 112-120, 2013.
KAUR, M.; KOHLI, S.; SANDHU, S.; BANSAL, Y.; BANSAL, G. Coumarin: a promising scaffold for anticancer agents. Anti-Cancer Agents in Medicinal Chemistry, v. 15, n. 8, p. 1032-1048, 2015.
KIM, T.W.; LEE, S.Y.; KIM, M.; CHEON, C.; KO, S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death & Disease, v. 9, n. 9, 875, 2018.
KIM, S.-H.; CHOO, G.-S.; YOO, E.-S.; WOO, J.-S.; HAN, S.-H.; LEE, J.-H.; JUNG, J.-Y. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncology Reports, v. 42, n. 5, p. 1904-1914, 2019.
KLEIN-JÚNIOR, L.C.; CAMPOS, A.; NIERO, R.; CORRÊA, R.; VANDER HEYDEN, Y.; FILHO, V.C. Xanthones and cancer: from natural sources to mechanisms of action. Chemistry & Biodiversity, v. 17, n. 2, e1900499, 2020.
KU, H.C.; LEE, S.Y.; YANG, K.C.; KUO, Y.H.; SU, M.J. Modification of caffeic acid with pyrrolidine enhances antioxidant ability by activating AKT/HO-1 pathway in heart. PLoS One, v. 11, n. 2, e0148545, 2016.
KROONEN, J.; ARTESI, M.; CAPRARO, V.; NGUYEN-KHAC, M.T.; WILLEMS, M.; CHAKRAVARTI, A.; BOURS, V.; ROBE, P.A. Casein kinase 2 inhibition modulates the DNA damage response but fails to radiosensitize malignant glioma cells. International Journal of Oncology, v. 41, n. 2, p. 776-782, 2012.
KUETE, V.; MBAVENG, A. T.; NONO, E. C. N.; SIMO, C. C.; ZEINO, M.; NKENGFACK, A. E.; EFFERTH, T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine, v. 23, n. 8, p. 856-863, 2016.
KY, I.; LE FLOCH, A.; ZENG, L.; PECHAMAT, L.; JOURDES, M.; TEISSEDRE, P.L. Encyclopedia of Food and Health. Elsevier: New York, p. 247–255, 2015.
LANG, S.J.; SCHMIECH, M.; HAFNER, S.; PAETZ, C.; WERNER, K.; EL GAAFARY, M.; SCHMIDT, C.Q.; SYROVETS, T.; SIMMET, T. Chrysosplenol d, a flavonol from Artemisia annua, induces ERK1/2-mediated apoptosis in triple negative human breast cancer cells. International Journal of Molecular Sciences, v. 21, n. 11, 4090, 2020.
LAPENNA, S.; GIORDANO, A. Cell cycle kinases as therapeutic targets for cancer. Nature Reviews Drug Discovery, v. 8, p. 547-566, 2009.
LEE, H.-L.; LIN, C.-S.; KAO, S.-H.; CHOU, M.-C. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anticancer Drugs, v. 28, n. 10, p. 1150-1156, 2017.
LEE, D.-Y.; YUN, S.-M.; SONG, M.-Y.; JUNG, K.; KIM, E.-H. Cyanidin chloride induces apoptosis by inhibiting NF-κB signaling through activation of Nrf2 in colorectal cancer cells. Antioxidants, v. 9, n. 4, 285, 2020.
LI, H.; LI, Z.; XU, Y.M.; WU, Y.; YU, K.K.; ZHANG, C.; JI, Y.H.; DING, G.; CHEN, F.X. Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neuroscience Bulletin, v. 30, n. 1, p. 67-73, 2014.
LI, W.; DU, Q.; LI, X.; ZHENG, X.; LV, F.; XI, X.; HUANG, G.; YANG, J.; LIU, S. Eriodictyol inhibits proliferation, metastasis and induces apoptosis of glioma cells via PI3K/Akt/NF-κB signaling pathway. Frontiers in Pharmacology, v. 11, 114, 2020.
LIN, H.-H.; CHEN, J.-H.; HUANG, C.-C.; WANG, C.-J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. International Journal of Cancer, v. 120, n. 11, p. 2306-2316, 2007.
LIN, M.-H.; CHENG, C.-H.; CHEN, K.-C.; LEE, W.-T.; WANG, Y.-F.; XIAO, C.-Q.; LIN, C.-W. Induction of ROS-independent JNK-activation-mediated apoptosis by a novel coumarin derivative, DMAC, in human colon cancer cells. Chemico-Biological Interactions, v. 218, p. 42-49, 2014.
LIN, J.; TIAN, J.; SHU, C.; CHENG, Z.; LIU, Y.; WANG, W.; LIU, R.; LI, B.; WANG, Y. Malvidin-3-galactoside from blueberry suppresses the growth and metastasis potential of hepatocellular carcinoma cell Huh-7 by regulating apoptosis and metastases pathways. Food Science and Human Wellness, v. 9, n. 2, p. 136-145, 2020.
LINDENMEYER, F.; LI, H.; MENASHI, S.; SORIA, C.; LU, H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutrition and Cancer, v. 39, n. 1, p. 139-147, 2001.
LIU, H.; ZENG, Z.; WANG, S.; LI, T.; MASTRIANI, E.; LI, Q.-H.; BAO, H.-X.; ZHOU, Y.-J.; WANG, X.; LIU, Y.; LIU, W.; HU, S.; GAO, S.; YU, M.; QI, Y.; SHEN, Z.; WANG, H.; GAO, T.; DONG, L.; JOHNSTON, R.N.; LIU, S.-L. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biology & Therapy, v. 18, n. 12, p. 990-999, 2017.
LIU, Q.; LUO, L.; ZHENG, L. Lignins: biosynthesis and biological functions in plants. International Journal of Molecular Sciences, v. 19, n. 2, 335, 2018.
LIU, J.; BAO, H.; WANG, H.; LUO, Q.; ZUO, J.; LIU, Z.; QIU, S.; SUN, X.; LIU, X. Synthesis of xanthone derivatives and anti-hepatocellular carcinoma potency evaluation: induced apoptosis. RSC Advances, v. 9, n. 70, p. 40781–40791, 2019.
LU, F.J.; CHU, L.H.; GAU, R.J. Free radical-scavenging properties of lignin. Nutrition and Cancer, v. 30, n. 1, p. 31-38, 1998.
LU, L.Y.; OU, N.; LU, Q.-B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Scientific Reports, v. 3, 3169, 2013.
LU, X.; LI, Y.; LI, X.; AISA, H.A. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncology Letters, v. 14, n. 2, p. 1993–2000, 2017.
LUCAS, J.; HSIEH, T.-C.; HALICKA, H.D.; DARZYNKIEWICZ, Z.; WU, J. Upregulation of PD-L1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300-mediated NF-κB signaling. International Journal of Oncology, v. 53, n. 4, p. 1469–1480, 2018.
MAGGIONI, D.; BIFFI, L.; NICOLINI, G.; GARAVELLO, W. Flavonoids in oral cancer prevention and therapy. European Journal of Cancer Prevention, v. 24, n. 6, p. 517-528, 2015.
MAITI, S.; ACHARYYA, N.; GHOSH, T. K.; ALI, S. S.; MANNA, E.; NAZMEEN, A.; SINHA, N. K. Green tea (Camellia sinensis) protects against arsenic neurotoxicity via antioxidative mechanism and activation of superoxide dismutase activity. Central Nervous System Agents in Medicinal Chemistry, v. 17, n. 3, 187-195, 2017.
MAJDALAWIEH, A.F.; MASSRI, M.; NASRALLAH, G.K. A comprehensive review on the anticancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). European Journal of Pharmacology, v. 815, p. 512-521, 2017.
MALI, A.V.; PADHYE, S.B.; ANANT, S.; HEGDE, M.V.; KADAM, S.S. Anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. European Journal of Pharmacology, v. 852, p. 107-124, 2019.
MANSOORI, B.; MOHAMMADI, A.; DAVUDIAN, S.; SHIRJANG, S.; BARADARAN, B. The different mechanisms of cancer drug resistance: A brief review. Advanced Pharmaceutical Bulletin, v. 7, n. 3, p. 339-348, 2017.
MARTINI, S.; CONTE, A.; TAGLIAZUCCHI, D. Anti-proliferative activity and cell metabolism of hydroxycinnamic acids in human colon adenocarcinoma cell lines. Journal of Agricultural and Food Chemistry, v. 67, n. 14, p. 3919-3931, 2019.
MARTINO, E.; VUOSO, D.C.; D’ANGELO, S.; MELE, L.; D’ONOFRIO, N.; PORCELLI, M.; CACCIAPUOTI, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Scientific Reports, v. 9, n. 1, 13045, 2019.
MAYNE, S.T.; PLAYDON, M. C.; ROCK, C. L. Diet, nutrition, and cancer: past, present and future. Nature Reviews. Clinical Oncology, v. 13, n. 8, p. 504-515, 2016.
MEMARIANI, Z.; ABBAS, S.Q.; UL HASSAN, S.S.; AHMADI, A.; CHABRA, A. Naringin and naringeninin as anticancer agents and adjuvants in cancer combination therapy; efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacological Research, 105264, 2020.
MENEZES, J. C.; DIEDERICH, M. Translational role of natural coumarins and their derivatives as anticancer agents. Future Medicinal Chemistry, v. 11, n. 9, p. 1057-1082, 2019.
MENSE, S.M.; HEI, T.K.; GANJU, R.K.; BHAT, H.K. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environmental Health Perspectives, v. 116, n. 4, p. 426-433, 2008.
MILES, E.A.; ZOUBOULI, P.; CALDER, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive identified in human whole blood cultures. Nutrition, v. 21, n. 3, p. 389-394, 2005.
MOHAMED, T. K.; BATRAN, R. Z.; ELSEGINY, S. A.; ALI, M.M.; MAHMOUD, A.E. Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis. Bioorganic Chemistry, v. 85, p. 253-273, 2019.
MOJZIS, J.; SARISSKÝ, M.; PILÁTOVÁ, M.; VOHAROVÁ, V.; VARINSKÁ, L.; MOJZISOVÁ, G.; OSTRO, A.; URDZÍK, P.; DANKOVCIK, R.; MIROSSAY, L. In vitro antiproliferative and antiangiogenic effects of Flavin7. Physiological Research, v. 57, n. 3, 413-420, 2008.
MONDAL, S.; RAHAMAN, S.T. Flavonoids: A vital resource in healthcare and medicine. Pharmacy & Pharmacology International Journal, v. 8, n. 2, p. 91-104, 2020.
MORENO-JIMÉNEZ, M. R.; LÓPEZ-BARRAZA, R.; CERVANTES-CARDOZA, V.; PÉREZ-RAMÍREZ, I. F.; REYNA-ROJAS, J. A.; GALLEGOS-INFANTE, J. A; ESTRELLA, I.; ROJAS-CONTRERAS, J. A.; GONZÁLEZ-LAREDO, R. F.; ROCHA-GUZMÁN, N. E. Mechanisms associated to apoptosis of cancer cells by phenolic extracts from two canned common beans varieties (Phaseolus vulgaris L.). Journal of Food Biochemistry, v. 43, n. 6, e12680, 2019.
MORSY, S.A.; FARAHAT, A.A.; NASR, M.N.A.; TANTAWY, A.S. Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharmaceutical Journal, v. 25, n. 6, p. 873-883, 2017.
MORTEZAEE, K.; SALEHI, E.; MIRTAVOOS- MAHYARI, H.; MOTEVASELI, E.; NAJAFI, M.; FARHOOD, B.; ROSENGREN, R.J.; SAHEBKAR, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. Journal of Cellular Physiology, v. 234, n. 8, p. 12537-12550, 2019.
MULLER, A. G.; SARKER, S. D.; SALEEM, I. Y.; HUTCHEON, G. A. Delivery of natural phenolic compounds for the potential treatment of lung cancer. Daru Journal of Pharmaceutical Sciences, v. 27, n. 1, p. 433-449, 2019.
MURTI, Y.; MISHRA, P. Synthesis and evaluation of flavanones as anticancer agents. Indian Journal of Pharmaceutical Sciences, v. 76, n. 2, p. 163-166, 2014.
NAINWAL, L.M.; ALAM, M.M.; SHAQUIQUZZAMAN, M.; MARELLA, A.; KAMAL, A. Combretastatin-based compounds with therapeutic characteristics: a patent review. Expert Opinion on Therapeutic Patents, v. 29, n. 9, p. 703-731, 2019.
NANI, B.D.; FRANCHIN, M.; LAZARINI, J.G.; FREIRES, I.A.; DA CUNHA, M.G.; BUENO-SILVA, B.; DE ALENCAR, S.M.; MURATA, R.M.; ROSALEN, P.L. Isoflavonoids from Brazilian red propolis down-regulate the expression of cancer-related target proteins: A pharmacogenomic analysis. Phytotherapy Research, v. 32, n. 4, p. 750–754, 2018.
NAUMAN, M.C.; TOCMO, R.; VEMU, B.; VEENSTRA, J.P.; JOHNSON, J.J. Inhibition of CDK2/CyclinE1 by xanthones from the mangosteen ( Garcinia mangostana ): a structure-activity relationship study. Natural Product Research, p. 1-5, 2020.
NARDINI, M.; PISU, P.; GENTILI. V.; NATELLA, F.; DI FELICE, M.; PICCOLELLA, E.; SCACCINI, C. Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress in U937. Free Radical Biology and Medicine, v. 25, n. 9, p. 1098-1105, 1998.
NARDINI, M.; SCACCINI, C.; PACKER, L.; VIRGILI, F. In vitro inhibition of the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin-rich pine bark (Pinus marittima) extract. Biochimica et Biophysica Acta, v. 1474, n. 2, p. 219-225, 2000.
NGUYEN, L. T.; LEE, Y. H.; SHARMA, A. R.; PARK, J. B.; JAGGA, S.; SHARMA, G.; LEE, S. S.; NAM, J. S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. The Korean Journal of Physiology & Pharmacology, v. 21, n. 2, p. 205-213, 2017.
NOEL, S.; KASINATHAN, M.; RATH, S.K. Evaluation of apigenin using in vitro cytochalasin blocked micronucleus assay. Toxicology in Vitro, v. 20, n. 7, p. 1168-1172, 2006.
OLIVEIRA, M. R.; NABAVI, S. F.; DAGLIA, M.; RASTRELLI, L.; NABAVI, S. M. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacological Research, v. 104, p. 70-85, 2016.
OSMAN, A.-M.M.; AL-MALKI, H.S.; AL-HARTHI, S.E.; EL-HANAFY, A.A.; ELASHMAOUI, H.M.; ELSHAL, M.F. Modulatory role of resveratrol on cytotoxic activity of cisplatin, sensitization and modification of cisplatin resistance in colorectal cancer cells. Molecular Medicine Reports, v. 12, n. 1, p. 1368-1374, 2015.
PAN, M.-H.; LIN, C.-C.; LIN, J.-K.; CHEN, W.-J. Tea polyphenol (-)-epigallocatechin 3-gallate suppresses heregulin-beta1-induced fatty acid synthase expression in human breast cancer cells by inhibiting phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase cascade signaling. Journal of Agricultural and Food Chemistry, v. 55, n. 13, p. 5030-5037, 2007.
PARI, L.; RASATH, A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chemico-Biological Interactions, v. 173, n. 2, p. 77-83, 2008.
PELINSON, L.P.; ASSMANN, C.E.; PALMA, T.V.; DA CRUZ, I.B.M.; PILLAT, M.M.; MÂNICA, A.; STEFANELLO, N.; WEIS, G.C.C.; DE OLIVEIRA ALVES, A.; DE ANDRADE, C.M.; ULRICH, H.; MORSCH, V.M.M.; SCHETINGER, M.R.C.; BAGATINI, M.D. Anti-proliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Molecular Biology Reports, v. 46, n. 2, p. 2085-2092, 2019.
PITKIN, J. Alternative and complementary therapies for the menopause. Menopause International, v. 18, n. 1, p. 20-27, 2012.
QAZI, S.S.; LI, D.; BRIENS, C.; BERRUTI, F.; ABOU-ZAID, M.M. Antioxidant activity of the lignins derived from fluidized-bed fast pyrolysis. Molecules, v. 22, n. 3, 372, 2017.
RAHAIEE, S.; ASSADPOUR, E.; ESFANJANI, A. F.; SILVA, A. S.; JAFARI, S. M. Application of nano/microencapsulated phenolic compounds against cancer. Advances in Colloid and Interface Science, v. 279, p. 102153, 2020.
RAUF, A.; IMRAN, M.; BUTT, M.S.; NADEEM, M.; PETERS, D.G.; MUBARAK, M.S. Resveratrol as an anticancer agent: A review. Critical Reviews in Food Science and Nutrition, v. 58, n. 9, p. 1428-1447, 2018.
REN, Q.-C.; GAO, C.; XU, Z.; FENG, L.-S.; LIU, M.; WU, X.; ZHAO, F. Bis-coumarin derivatives and their biological activities. Current Topics in Medicinal Chemistry, v. 18, n. 2, p. 101-113, 2018.
ROGERS, L.A.; CAMPBELL, M.M. The genetic control of lignin deposition during plant growth and development. New Phytologist, v. 164, n. 1, p. 17-30, 2004.
RUAN, J.; ZHENG, C.; LIU, Y.; QU, L.; YU, H.; HAN, L.; ZHANG, Y.; WANG, T. Chemical and biological research on herbal medicines rich in xanthones. Molecules, v. 22, n. 10, 1698, 2017.
RYU, S.; LIM, W.; BAZER, F.W.; SONG, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. Journal of Cellular Physiology, v. 232, n. 12, p. 3786–3797, 2017.
SAIDU, N.E.B.; VALENTE, S.; BANA, E.; KIRSCH, G.; BAGREL, D.; MONTENARH, M. Coumarin polysulfides inhibit cell growth and induce apoptosis in HCT116 colon cancer cells. Bioorganic & Medicinal Chemistry, v. 20, n. 4, p. 1584-1593, 2012.
SALMANI, J.M.M.; ZHANG, X.-P.; JACOB, J.A.; CHEN, B.-A. Apigenin’s anticancer properties and molecular mechanisms of action: Recent advances and future prospectives. Chinese Journal of Natural Medicines, v. 15, n. 5, p. 321–329, 2017.
SÁNCHEZ-CARRANZA, J.; ALVAREZ, L.; MARQUINA-BAHENA, S.; SALAS-VIDAL, E.; CUEVAS, V.; JIMÉNEZ, E.; VELOZ G.R.; CARRAZ, M.; GONZÁLEZ-MAYA, L. Phenolic compounds isolated from Caesalpinia coriaria induce S and G2/M phase cell cycle arrest differentially and trigger cell death by interfering with microtubule dynamics in cancer cell lines. Molecules, v. 22, n. 4, p. 666, 2017.
SELMA, M. V.; ESPÍN, J. C.; TOMÁS-BARBERÁN, F. A. Interaction between phenolics and gut microbiota: role in human health. Journal of Agricultural and Food Chemistry, v. 57, p. 6485-6501, 2009.
SIDDIQUI, L.; MISHRA, H.; MISHRA, P.K.; IQBAL, Z.; TALEGAONKAR, S. Novel 4-in-1 strategy to combat colon cancer, drug resistance and cancer relapse utilizing functionalized bioinspiring lignin nanoparticle. Medical Hypotheses, v. 121, p. 10-14, 2018.
SIEMANN, D.W.; CHAPLIN, D.J.; WALICKE, P.A. A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opinion on Investigational Drugs, v. 18, n. 2, p. 189-197, 2009.
SIENIAWSKA, E. Activities of tannins – from in vitro studies to clinical trials. Natural Product Communications, v. 10, p. 1877–1884, 2015.
SILVA, J. P.; GOMES, A. C.; COUTINHO, O. P. Oxidative DNA damage protection and repair by polyphenolic compounds in PC12 cells. European Journal of Pharmacology, v. 601, n. (1-3), p. 50-60, 2008.
SILVA, F.; ALCORN, J. flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: Chemistry, pharmacokinetics, and molecular targets. Pharmaceuticals (Basel), v. 12, n. 2, 68, 2019.
SIMÕES, C.M.O.; SCHENKEL, E.P.; GOSMANN, G.; DE MELLO, J.C.P.; MENTZ, L.A.; PETROVICK, P.R. Farmacognosia: da planta ao medicamento, 6ª ed. UFSC/UFRGS: Porto Alegre, 2007.
SINGH, S.; SHARMA, B.; KANWAR, S.S.; KUMAR, A. Lead phytochemicals for anticancer drug development. Frontiers in Plant Science, v. 7, p. 1667, 2016.
SINGH, S. K.; BANERJEE, S.; ACOSTA, E. P.; LILLARD, J. W.; SINGH, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget, v. 8, n. 10, p. 17216–17228, 2017.
SITAREK, P.; SKAŁA, E.; TOMA, M.; WIELANEK, M.; SZEMRAJ, J.; SKORSKI, T.; BIAŁAS, A.J.; SAKOWICZ, T.; KOWALCZYK, T.; RADEK, M.; WYSOKIŃSKA, H.; ŚLIWIŃSKI, T. Transformed root extract of leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathology & Oncology Research, v. 23, n. 3, p. 679-687, 2017.
SOUSA, E.P.; SILVA, A.M.S.; PINTO, M.M.M.; PEDRO, M.M.; CERQUEIRA, F.A.M.; NASCIMENTO, M.S.J. Isomeric kielcorins and dihydroxyxanthones: synthesis, structure elucidation, and inhibitory activities of growth of human cancer cell lines and on the proliferation of human lymphocytes in vitro. Helvetica Chimica Acta, v. 85, n. 9, p. 2862-2876, 2002.
SOUSA MORAES, L.F.; SUN, X.; PELUZIO, M.C.G.; ZHU, M.J. Anthocyanins/anthocyanidins and colorectal cancer: What is behind the scenes? Critical Reviews in Food Science and Nutrition, v. 59, n. 1, p. 59-71, 2019.
STEFANACHI, A.; LEONETTI, F.; PISANI, L.; CATTO, M.; CAROTTI, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules, v. 23, n. 2, 250, 2018.
STEVENS, Y.; RYMENANT, E.V.; GROOTAERT, C.; CAMP, J.V.; POSSEMIERS, S.; MASCLEE, A.; JONKERS, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients, v. 11, n. 7, 1464, 2019.
STEWARD, W. P.; BROWN, K. Cancer chemoprevention: a rapidly evolving field. British Journal of Cancer, v. 109, n. 1, p. 1-7, 2013.
SUBBARAMAIAH, K.; SUE, E.; BHARDWAJ, P.; DU, B.; HUDIS, C. A.; GIRI, D.; KOPELOVICH, L.; ZHOU, X. K.; DANNENBERG, A. J. Dietary polyphenols suppress elevated levels of proinflammatory mediators and aromatase in the mammary gland of obese mice. Cancer Prevention Research, v. 6, n. 9, p. 886-897, 2013.
SUGAHARA, T.; YAMAUCHI, S.; NISHIMOTO, S.; KONDO, A.; OHNO, F.; TOMINAGA, S.; NAKASHIMA, Y.; KISHIDA, T.; AKIYAMA, K.; MARUYAMA, M.; KAKINUMA, Y. The structure-activity relationships offlaxseed lignan, secoisolariciresinol. In: Interdisciplinary studies on environmental chemistry—biological responses tochemical pollutants. MURAKAMI, Y.; NAKAYAMA, K.; KITAMURA, S.I.; IWATA, H.; TANABE, S., Eds.; Terrapub: Tokyo, v. 1, p. 263–268, 2008.
SUN, Y.; ZHOU, Q. M.; LU, Y. Y.; ZHANG, H.; CHEN, Q. L.; ZHAO, M.; SU, S. B. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules, v. 24, n. 6, p. 1131, 2019.
SURH, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer, v. 3, n. 10, p. 768-780, 2003.
TANG, Y.; LI, X.; LIU, Z.; SIMONEAU, A.R.; XIE, J.; ZI, X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. International Journal of Cancer, v. 127, n. 8, p. 1758-1768, 2010
TANG, Q.; LU, M.; ZHOU, H.; CHEN, D.; LIU, L. Gambogic acid inhibits the growth of ovarian cancer tumors by regulating p65 activity. Oncology Letters, v. 13, n. 1, p. 384-388, 2017.
TAO, S.-F.; HE, H.-F.; CHEN, Q. Quercetin inhibits proliferation and invasion acts by upregulating miR-146a in human breast cancer cells. Molecular and Cellular Biochemistry, v. 402, n. 1-2, p. 93-100, 2015.
THAKUR, A.; SINGLA, R.; JAITAK, V. Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. European Journal of Medicinal Chemistry, v. 101, p. 476-495, 2015.
TUNGMUNNITHUM, D.; THONGBOONYOU, A.; PHOLBOON, A.; YANGSABAI, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines, v. 5, n. 3, 93, 2018.
VELU, G.; PALANICHAMY, V.; RAJAN, A.P. Phytochemical and Pharmacological Importance of Plant Secondary Metabolites in Modern Medicine. In: Bioorganic Phase in Natural Food: An Overview. Springer International Publishing, Cham., p. 135–156, 2018.
VENTURELLI, S.; BURKARD, M.; BIENDL, M.; LAUER, U.M.; FRANK, J.; BUSCH, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition, v. 32, n. 11-12, p. 1171-1178, 2016.
VERVANDIER-FASSEUR, D.; LATRUFFE, N. The potential use of resveratrol for cancer prevention. Molecules, v. 24, n. 24, 4506, 2019.
WANG, B.; ZHAO, X.-H. Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma HCT-116 cells. Oncology Reports, v. 37, n. 2, p. 1132-1140, 2017.
WANG, Y.; REN, F.; LI, B.; SONG, Z.; CHEN, P.; OUYANG, L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. Journal of Cancer, v. 10, n. 15, p. 3303-3314, 2019.
WU, F.; CUI, L. Resveratrol suppresses melanoma by inhibiting NF-κB/miR-221 and inducing TFG expression. Archives of Dermatological Research, v. 309, n. 10, p. 823-831, 2017.
WU, S.; ZHU, W.; THOMPSON, P.; HANNUN, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nature Communications, v. 9, n. 1, p. 3490, 2018.
WRIGHT, C.; IYER, A. K. V.; YAKISICH, J. S.; AZAD, N. Anti-tumorigenic effects of resveratrol in lung cancer cells through modulation of c-FLIP. Current Cancer Drug Targets,v. 17, n. 7, p. 669-680, 2017.
XIANG, L.-P.; WANG, A.; YE, J.-H.; ZHENG, X.-Q.; POLITO, C.A.; LU, J.-L.; LI, Q.-S.; LIANG, Y.-R. Suppressive effects of tea catechins on breast cancer. Nutrients, v. 8, n. 8, 458, 2016.
XIE, L.; JIANG, F.; ZHANG, X.; ALITONGBIEKE, G.; SHI, X.; MENG, M.; XU, Y.; REN, A.; WANG, J.; CAI, L.; ZHOU, Y.; XU, Y.; SU, Y.; LIU, J.; ZENG, Z.; WANG, G.; ZHOU, H.; CHEN, Q.C.; ZHANG, X. Honokiol sensitizes breast cancer cells to TNF-α induction of apoptosis by inhibiting Nur77 expression. British Journal of Pharmacology, v. 173, n. 2, p. 344-356, 2016.
XU, D.P.; LI, Y.; MENG, X.; ZHOU,T.; ZHOU,Y.; ZHENG, J.; ZHANG, J.J.; LI, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. International Journal of Molecular Sciences, v. 18, n. 1, 96, 2017.
XU, H.; HU, M.; LIU, M.; AN, S.; GUAN, K.; WANG, M.; LI, L.; ZHANG, J.; LI, J.; HUANG, L. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials, v. 235, 119769, 2020.
YAN, T.; LI, D.; LI, J.; CHENG, F.; CHENG, J.; HUANG, Y.; HE, J. Effective co-delivery of doxorubicin and curcumin using a glycyrrhetinic acid-modified chitosan-cystamine-poly(ε-caprolactone) copolymer micelle for combination cancer chemotherapy. Colloids and Surfaces B: Biointerfaces, v. 145, p. 526-538, 2016.
YANG, J.; LIU, J.; LYU, X.; FEI, S. Resveratrol inhibits cell proliferation and upregulates MICA/B expression in human colon cancer stem cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, v. 31, n. 7, p. 889-893, 2015.
YANG, J.; PEI, H.; LUO, H.; FU, A.; YANG, H.; HU, J.; ZHAO, C.; CHAI, L.; CHEN, X.; SHAO, X.; WANG, C.; WU, W.; WAN, L.; YE, H.; QIU, Q.; PENG, A.; WEI, Y.; YANG, L.; CHEN, L. Non-toxic dose of liposomal honokiol suppresses metastasis of hepatocellular carcinoma through destabilizing EGFR and inhibiting the downstream pathways. Oncotarget, v. 8, n. 1, p. 915–932, 2017.
YIN, S.T.; TANG, M.L.; SU, L.; CHEN, L.; HU, P.; WANG, H.L.; RUAN, D.-Y. Effects of epigallocatechin-3 gallate on lead-induced oxidative damage. Toxicology, v. 249, p. 45-54, 2008.
YIN, L.; YU, X. Arsenic-induced apoptosis in the p53-proficient and p53-deficient cells through differential modulation of NFkB pathway. Food and Chemical Toxicology, v. 118, p. 849-860, 2018.
YOON, J.; CHOI, H.; AN, G. Roles of lignin biosynthesis and regulatory genes in plant development. Journal of Integrative Plant Biology, v. 57, n. 11, p. 902-912, 2015.
ZENG, Z.; LIN, C.; WANG, S.; WANG, P.; XU, W.; MA, W.; WANG, J.; XIANG, Q.; LIU, Y.; YANG, J.; YE, F.; XIE, K.; XU, J.; LUO, Y.; LIU, S.-L.; LIU, H. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine, 26, 153267, 2020.
ZHANG, J.-X.; XING, J.-G.; WANG, L.-L.; JIANG, H.-L.; GUO, S.-L.; LIU, R. Luteolin inhibits fibrillary β-amyloid1–40-induced inflammation in a human blood-brain barrier model by suppressing the p38 MAPK-mediated NF-κB signaling pathways. Molecules, v. 22, n. 3, 334, 2017.
ZHANG, L.; XU, Z. Coumarin-containing hybrids and their anticancer activities. European Journal of Medicinal Chemistry, v. 181, 111587, 2019.
ZHANG, J.; TAN, Y.; LI, G.; CHEN, L.; NIE, M.; WANG, Z.; JI, H. Coumarin sulfonamides and amides derivatives: design, synthesis, and antitumor activity in vitro. Molecules, v. 26, n. 4, 786, 2021.
ZHONG, R.; LEE, C.; MCCARTHY, R.L.; REEVES, C.K.; JONES, E.G.; YE, Z.H. Transcriptional activation of secondary wall biosynthesis by rice maize NAC and MYB transcription factors. Plant and Cell Physiology, v. 52, n. 10, p. 1856-1871, 2011.
ZIAEI, S.; HALABY, R. Dietary isoflavones and breast cancer risk. Medicines (Basel), v. 4, n. 2, 18, 2017.
[1] Master in Health Sciences (2019), Specialist conferred by AMB in Family and Community Medicine (2017) and Traffic Medicine; specialization by ABRAN in nutrology (2012). Graduated in Medicine from the José Rosário Velano University UNIFENAS -BH (2009). ORCID: 0000-0001-8094-9811.
[2] PhD student in Food Science at the Federal University of Lavras-UFLA. Master in Health Sciences from UFLA (2019). Bachelor of Nutrition from UFLA (2015). ORCID: 0000-0002-4209-3140.
[3] PhD in Agrochemistry from the Federal University of Lavras-UFLA (2020). Master in Agrochemistry from UFLA (2016). Graduated in Biological Sciences from UFLA (2012). ORCID: 0000-0003-3271-7797.
[4] PhD in Agrochemistry from the Federal University of Lavras- UFLA (2021). Master in Agrochemistry from UFLA (2017). Graduated in Biological Sciences from UFLA (2014). ORCID: 0000-0001-8463-9052.
[5] Post-Doctoral Student in Agrochemistry at the Federal University of Lavras- UFLA. PhD in Agrochemistry from the Federal University of Lavras- UFLA (2018). Master in Agrochemistry from UFLA (2015). Degree in Chemistry from UFLA (2012). ORCID: 0000-0003-0758-3993.
[6] Postdoctoral fellow in Neuroendocrinology of Female Reproduction at the Department of Physiology of the Faculty of Medicine of Ribeirão Preto – USP (FMRP-USP). FMRP-USP, Specialization in Human and Exercise Physiology and graduation in Biomedicine. He holds a Doctorate and Master’s degree in Physiology from FMRP-USP. ORCID: 0000-0002-2620-0081.
[7] Advisor. Post-doctorate in Pharmacology at FCFRP-USP (Department of Clinical, Toxicological and Bromatological analyses). PhD in Sciences from FMRP-USP (Department of Biochemistry and Immunology). Master in Biotechnology from UNAERP (2003). Degree in Biology from the Barão de Mauá Educational Organization (2001). ORCID: 0000-0002-4674-6911.
Sent: November, 2021.
Approved: August, 2022.