ORIGINAL ARTICLE
FERREIRA, Fernanda Amorim [1], PARAÍBA, Isabella Maria Rios [2], ELIAS, Laíse de Souza [3]
FERREIRA, Fernanda Amorim. PARAÍBA, Isabella Maria Rios. ELIAS, Laíse de Souza. Effect of melatonin on cardiac morphophysiology of rats induced hyperlipidemia. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 10, Vol. 13, pp. 130-143. October 2020. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/nutrition/effect-of-melatonin
SUMMARY
Hyperlipidemias are classified as metabolic alterations and are related to cardiovascular diseases, which highlight coronary athertotic disease. Cardiovascular diseases have a remarkable participation in mortality rates worldwide and, as a result, it has been the target of research that seeks therapeutic innovation. Melatonin is a hormone produced in an environmental phase of darkness by the pineal gland that plays antioxidant, lipid-lowering, anti-inflammatory function, among others, thus demonstrating to be a potent molecule in the treatment of cardiovascular diseases. Thus, the objective of this work is to evaluate the effect of melatonin on oxidative stress and biochemical parameters in rats induced by hyperlipidemia. For this, 15 male albino rats (Rattus norvegicus albinus) were used, with 150 days of age, coming from the bioterium of the department of Morphology and Animal Physiology of the Federal Rural University of Pernambuco. These animals were kept in cages, with feeding and water ad libitum, temperature of 22° C and artificial lighting that will establish a photoperiod of 12 hours light and 12 hours dark (inverted cycle). In conclusion, studies have shown that melatonin has effects on reducing lipid peroxidation, creatine kinase (CK) levels as well as reducing reduced glutathione. As a cheap and well-tolerated drug, melatonin may be a new therapeutic option for cardiovascular diseases and individuals with hyperlipidemia.
Keywords: Atherosclerosis, melatonin, heart, hyperlipidemia, oxidative stress.
INTRODUCTION
Hyperlipidemias are metabolic alterations that occur when circulating lipid levels exceed the limits of normality in the bloodstream (BEVILACQUA et al., 2007). This unregulated production can cause serious problems, including cardiovascular diseases (CVD), such as hypertension, atherosclerosis, in addition to damage to different organs and tissues, thus characterizing a serious public health problem (EMET et al., 2016).
CVDs have a remarkable participation in mortality rates worldwide and, as a result, has been the target of research that seeks therapeutic innovations. (SIMKO et al., 2016). Thus, in recent decades, the search for new drugs for the treatment of hyperlipidemia has valued the role of substances that act in controlling the increase in lipid profile and inflammation, in addition to protecting the vascular endothelium against oxidative stress (JOCKERS et al., 2016). Among these substances, melatonin has received much repercussion for the treatment of lesions in various organs, including the heart (YANG et al., 2014).
Melatonin (N-acetyl-5-methoxytryptaline) (MEL) is a neuroendocrine hormone produced by the pineal gland (HARDELAND et al., 2006). Its secretion is regulated by the environmental light/darkness cycle through the suprachiasmatic nucleus (SKENE et al., 2006). Since its biosynthesis began at night, from retinal photoreceptors that send signal to the pineal gland, stimulating the production and release of melatonin (GOOLEY et al., 2003). The mechanism of action of MEL depends on membrane receptors called MT1, MT2 and MT3, which are expressed both individually and together in various tissues and organs, the first two being expressed both in the heart and in the rat artery (CAHILL; GRACE; BESHARSE, 1991) (LI; ZHANG; TANG, 2013).
By presenting receptors widely distributed by the body, MEL can be responsible for a series of physiological events (FARÍAS; ZEPEDA; CALAF, 2012). Initially its function was restricted to the regulation of the sleep/wake cycle (SUN; Huang, Huang, QU, 2015) however, over the years other functions have also been reported, such as its antioxidant and anti-inflammatory role, in the regulation of lipid and glucose metabolism,(AGIL et al., 2011) (ESPINO; PARIENTE; RODRIGUEZ, 2011) as well as its cardioprotective effect, having an important role on heart disease (TAN et al., 1998), such as myocardial ischemia-reperfusion injury, atherosclerosis, hypertension and heart failure. (FAVERO et al., 2012) (BENOVA et al., 2013) (DOMINGUEZ; ABREU-GONZALEZ; REITER, 2014).
Studies in rats found that MEL produced a cardioprotective effect against cardiotoxicity induced by chemotherapeutic agents and recently the amplitude of the cardiac protective effect exercised by MEL (SUN; GUSDON; QU, 2016). In addition, melatonin was found to be efficient in reducing the effects of induced ischemia on the hearts of rats (YU et al., 2014), lowering heart rate, reducing creatine kinase protein levels, (FAVERO et al., 2016), preservation of the cardiomyocyte microstructure (LIN et al., 2018), reduction of cardiac arrhythmias and lipid oxidation resulting from cardiac ischemic processes( TARE et al., 2014), in addition to improving cardiac function and blood flow (ZHANG et al., 2017).
In view of the increasing increase in hyperlipidemia (KANG; KOH; LEE, 2011) and consequently cardiovascular diseases (HARDELAND, 2015) and that melatonin plays antioxidant function, (PANDI-PERUMAL et al., 2013) (COMMENTZ; HELMKE, 1995), (REITER et al., 2013) anti-inflammatory and lipid-lowering (ALLEGRA et al., 2003), it is necessary to study the efficacy of the effect of melatonin on cardiac morphophysiology in rats induced by hyperlipidemia. Thus, this research can contribute to the development of complementary therapeutic methods in the treatment of hyperlipidemia, as well as cardiovascular diseases. The present study aimed to evaluate the effect of melatonin on cardiac morphophysiology of rats induced to hyperlipidemia.
1. MATERIALS AND METHODS
1.1 EXPERIMENTAL GROUPS
We used 15 male albino rats (Rattus norvegicus albinus), 150 days old, from the bioterium of the Department of Morphology and Animal Physiology of the Federal Rural University of Pernambuco. These animals were kept in cages, with feeding and water ad libitum, temperature of 22° C and artificial lighting that established a photoperiod of 12 hours light and 12 hours dark (inverted cycle). The animals were weighed and randomly divided into three experimental groups: GC: rats without induction of hyperlipidemia; GI: rats induced to hyperlipidemia and treated with placebo; GM: rats induced to hyperlipidemia and treated with melatonin.
1.2 INDUCTION OF HYPERLIPIDEMIA
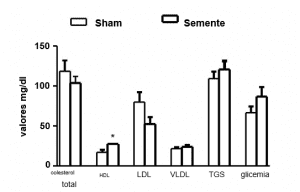
To induce hyperlipidemia, Triton WR 1339 was administered intraperitoneally for thirty days, also known as tyloxapol, a non-anionic detergent of polymeric structure, at a dose of 400mg/kg of body weight, dissolved in NaCl at 0.9%. This substance has been used in experimental studies because it is capable of causing hyperlipidemia in animals. Biochemical analyses were used to follow up hyperlipidemia after the third day of induction, according to Labtest® kits, following the specified catalogues: LDL, HDL, triglycerides and total cholesterol. Animals not induced to hyperlipidemia (GC) received equivalent doses of saline solution in the same way.
1.3 MELATONIN ADMINISTRATION
Melatonin was administered orally (VO), so 10mg/kg of melatonin was dissolved in 200 microliters of alcohol. Then, at 7:00 a.m., this solution was diluted in the drinking water of the animals, it is worth noting that the bottles were coated with aluminum foil to protect the luminosity content.
1.4 BIOCHEMICAL ANALYSIS
Blood samples were collected in the periods before induction and after treatment. For analysis before the induction period, the rats were mobilized by mechanical containment and the blood collected by lateral caudal puncture with the use of catheter (24G). For analysis after induction and its respective treatment, blood was collected by cardiac puncture. The material was centrifuged, the overnnatant packed in Eppendorf and kept in a freezer at -20°c until the time of dosages. For this purpose, the Labtest® kit was used, following the specific catalogue: CK protein (133-1/500).
1.5 OXIDATIVE STRESS ANALYSIS: LIPID PEROXIDATION AND REDUCED GLUTATHIONE
Lipid peroxidation was estimated by measuring the levels of thiobarbituric acid reactive substances (TBARS), while reduced glutathione (GSH) was determined by measuring non-protein sulfhydron groups. For this, fragments of the heart were macerated in KCl, 1.15% in a proportion of 10 ml/1g until complete homogenization of the collected material. The homogenate was transferred to a test tube, to which 2mL of regent (0.375% thiobarbituric acid and 75% trichloroacetic acid) was added to each mL of the mixture. The duplicate tubes were sealed and heated in a water bath (100 °C) for 15 minutes. The overnum was separated and absorbance measured at 535 nm.
1.6 ETHICAL PROCEDURES
The experiment was developed in accordance with the Ethical Principles in Animal Experimentation, according to Law No. 11,794 of October 8, 2008, and the project was approved by the Ethics Commission on the Use of Animals (CEUA) of the Federal Rural University of Pernambuco (UFRPE – process number 23082.015842/2014) (ANNEX A).
1.7 STATISTICAL ANALYSIS
Statistical analyses were performed using the Kruskal-Wallis nonparametric method, where the means were compared by the Wilcoxon-Mann Whitney test, 95% of significance.
2. RESULTS AND DISCUSSION
The oxidative damage induced in cells and tissues has been related to physiological processes and to the etiology of various diseases, such as aging, and chronic diseases such as diabetes, cancer and atherosclerosis, where it is often observed the increase in the content of the most important reactive oxygen species (OSIs) in the biological context (ROSEN et al., 2001) (HIGASSHI et al., 2014).
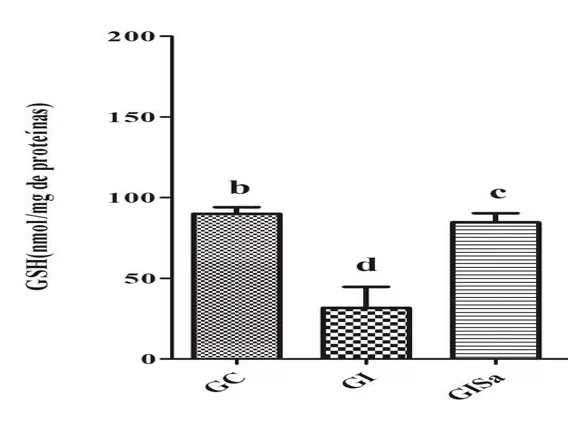
Analyses of TBARS levels in cardiac tissue after induction to hyperlipidemia revealed high values when compared to the control group. However, melatonin treatment attenuated TBARs levels, presenting values similar to GC (Figure 1). The tissue analysis of GSH levels in the group induced and treated with melatonin were superior to the animals of the GI group, also demonstrating values similar to those of the control group (Figure 2). With this, we realized that melatonin was able to reduce oxidative damage.
This hormone has the ability to kidnap reactive oxygen (EROs) and nitrogen (NRE) species, acting to prevent oxidative damage (ALVES et al., 2014) in addition to stimulating the expression of antioxidant enzymes (PANDI-PERUMAL et al., 2013) through the growth of the expression of the Keap1-Nrf2 (MANCHESTER et al., 2015).
ONK (2016) found that the use of 20mg/kg of melatonin resulted in decreased Levels of TBARS. Similarly, Debosree Ghosh (2015) showed that the minimum dose of 10 mg/kg of melatonin was necessary to protect against increased lipid peroxidation in the red blood cells of rats.
Numerous studies have been reported demonstrating the isolated effect of melatonin on the promotion of GSH levels, e.g., increased Levels of GSH have been demonstrated in animals with thioacetamide-induced liver damage (TAA) and treated with melatonin (10 mg/kg) Czechowska (2015) and Goc (2017), observed high levels of GSH in the liver tissue of animals treated with 10 mg/kg of MEL alone or in combination with sodium nitroprusside (SNP), an antihypertensive drug with toxic effects, compared to the isolated SNP administration.
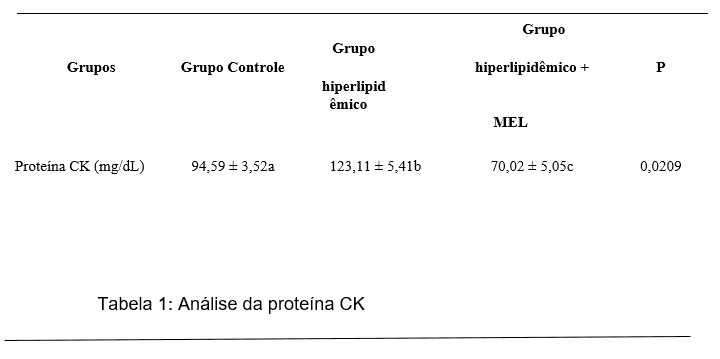
In our study, the group induced to hyperlipidemia and treated with melatonin, demonstrated a reduction in Ck protein levels compared to the hyperlipidemic group (GI), however, in relation to the control group, no significant alteration was observed (Table 1).
Creatine kinase (CK) is an enzyme that plays an important role in generating energy for muscle metabolism. It is present predominantly in muscle tissue, but is also found in brain tissue (BRANCACCIO et al., 2008). Among the molecules used as a marker of cardiac damage, creatine kinase (CK) is often described as the best indirect marker of damage to muscle tissue (FOSCHINI et al., 2017). This reduction in CK levels can be attributed to the membrane stabilization effects given by MEL. In vitro studies show that MEL is able to inhibit the internalization of phosphatidylserine, Bax expression and xanthine oxidase, thus causing stabilization of the lipid bilayer (KOCIC et al., 2017).
Ghaeli (2015), reports that in patients with myocardial infarction with ST-segment overleveling submitted to primary percutaneous coronary intervention, melatonin administration plus standard treatment considerably decreased creatine kinase-MB level. In addition, MEL still has anti-inflammatory and anti-apoptotic effects, thus being able to act in the control of cardiovascular diseases (CHEN et al., 2016).
3. CONCLUSION
In conclusion, studies have shown that melatonin has effects on reducing lipid peroxidation, creatine kinase (CK) levels as well as increasing reduced glutathione in hyperlipidemia-induced rats.
REFERENCES
AGIL, A, Navarro-Alarcon M, Ruiz R, et al. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res 2011; 50:207–212.
ALLEGRA, M.; REITER, R. J.; TAN, D. X.; et al. The chemistry of melatonin’s interaction with species. J. Pineal Res., v.34, n.1, p. 1–10, 2003.
ALVES, R.S.C. et al. A melatonina e o sono em crianças. Pediatria. v. 1, p. 587-594,2004.
BRANCACCIO, P.; Maffulli, N.; Buonauro, R. Serum enzyme monitoring in sports medicine. Clin Sports Med 2008. 27:1-1.
BENOVA, T, Viczenczova C, Radosinska J, et al. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can J Physiol Pharmacol 2013; 91:633–639.
BEVILACQUA, MR, GIMENO SGA, BEVILACQUA MR, Matsumura LK, et al. Hiperlipidemias e Fatores Dietéticos: Estudo Transversal Entre Nipo-Brasileiros. Arq Bras Endocrinol Metab. 2007 mar/out; 51/4: 547-558.
CAHILL, GM, Grace MS, Besharse JC. Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol Neurobiol 1991; 11:529–560.
CHEN, S,J. et al. Melatonin enhances interleukin-10 expression and suppresses chemotaxis to inhibit inflammation in situ and reduce the severity of experimental autoimmune encephalomyelitis. Int Immunopharmacol. v.31, p.169-77, 2016.
COMMENTZ, J. C.; HELMKE, K. Precocious puberty and decreased melatonin1583 secretion due to a hypothalamic hamartoma. Horm.Res. v.44, n. 6, p. 271-275,1584 1995.
CZECHOWSKA, G. et al. Protective effects of melatonin against thioacetamide induced liver fibrosis in rats. J Physiol Pharmacol. v.66, p.567-79, 2015.
DEBOSREE Ghosh; Sudeshna Paul, Aindrila Chattopadhyay, Debasish Bandyopadhy. Melatonin and aqueous curry leaf extract in combination protects against lead induced oxidative stress mediated injury to rat heart: a new approach. Journal of Pharmacy Research 2015,9(12),618-634.
DOMINGUEZ -Rodriguez A, Abreu-Gonzalez P, Reiter RJ. The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int J Cardiol 2014; 174:415–417.
EMET M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A. A review of melatonin, its receptors and drugs. Eur J Med. 2016; 48(2):135-41.
ESPINO J, Pariente JA, Rodriguez AB. Role of melatonin on diabetes-related metabolic disorders. World J Diabetes 2011; 2:82–91.
FARIAS JG, Zepeda AB, Calaf GM. Melatonin protects the heart, lungs and kidneys from oxidative stress under intermittent hypobaric hypoxia in rats. Biol Res. 2012; 45(1):81-5.
FAVERO G, Rodella LF, Reiter RJ, et al. Melatonin and its atheroprotective effects: a review. Mol Cell Endocrinol 2014; 382:926–937.
FAVERO G, Franceschetti L, Buffoli B, Moghadasian MH, Reiter RJ, Rodella LF, et al. Melatonin: Protection against age-related cardiac pathology. Ag Res Rev. 2016; 22: S1568-1637. 16.
FOSCHINI D, Prestes J, Charro MA. Relação entre exercício físico, dano muscular e dor muscular de início tardio. Rev Bras Cineantropom Desempenho Hum 2007;9(1):101-6.
GHAELI P, Vejdani S, Ariamanesh A, et al. Effect of melatonin on cardiac injury after primary percutaneous coronary intervention: a randomized controlled trial. Iran J Pharm Res 2015; 14:851–855.
GOC, Z. et al. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin J Physiol. v.1, p. 28-34 2017.
GOOLEY, JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci 2003; 23:7093–7106.
HARDELAND, R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006; 38:313–316.
HARDELAND, R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. v. 27, p. 111-118, 2015.
HIGASSHI; T Maruhashi, K Noma, Y Kihara Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications, Trends in Cardiovascular Medicine Volume 24, Issue 4, May 2014, Pages 165-169.
JOCKERS, R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. Update on melatonin receptors: IUPHAR Review. Br J Pharmacology. 2016; 173:2702-25.
KANG, J. W.; KOH, E. J.; LEE, S. M. Melatonin protects liver against ischemia and reperfusion injury through inhibition of toll-like receptor signaling pathway. J Pineal Res. v.50, p. 403-411, 2011.
KOCIC, G., Tomovic, K., Kocic, H., et al., 2017. Antioxidative, membrane protective and antiapoptotic effects of melatonin, in silico study of physico-chemical profile and efficiency of nanoliposome delivery compared to betaine. RSC Adv. 7, 1271–1281.
LI, X.; ZHANG, M.; TANG, W. Effects of melatonin on streptozotocin-induced retina neuronal apoptosis in high blood glucose rat. Neurochem Res. v.38, p.669- 76, 2013.
LIN, X, Zhao T, Lin CH et al. Melatonin provides protection against heat strokeinduced myocardial injury in male rats. J Pharm Pharmacol. 2018 Jun;70(6):760-767.
MANCHESTER LC, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. v.59, p. 403-19, 2015
ONK D, et al. Melatonin Attenuates Contrast-Induced Nephropathy in Diabetic Rats: The Role of Interleukin-33 and Oxidative Stress. Mediators Inflamm. v.2016, p. 508-28, 2016.
PANDI-PERUMAL, S. R. et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. v.23, p.267-300, 2013.
REITER, R. J. et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans – Acta Biochim. Pol.v. 50, p. 1129-1146, 2003.
RODRIGUEZ C, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9.
ROSEN, P P Nawroth, G King, W Möller, H J Tritschler, L Packer. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society – May-Jun 2001;17(3):189-212.
SIMKO F, Baka T, Paulis L, Reiter RJ. Elevated heart rate and non dripping heart rate as potential targets for melatonin: a review. J Pineal Res. 2016; 61:127-37.
SKENE, DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006; 43:344–353.
SUN H, Huang FF, Qu S. Melatonin: a potential intervention for hepatic steatosis. Lipids Health Dis. 2015; 14:75.
SUN H, Gusdon AM, Qu S. Effects of melatonin on cardiovascular diseases: progress in the past year. Curr Opin Lipidol. 2016; 27(4):408-13.
TAN DX, Manchester LC, Reiter RJ, et al. Ischemia/reperfusion-induced arrhythmias in the isolated rat heart: prevention by melatonin. J Pineal Res. 1998; 25:184–191.
TARE M, Parkington HC, Wallace EM et al. Maternal melatonin administration mitigates coronary stiffness and endothelial dysfunction, and improves heart resiliência to insult in growth restricted lambs. 2014
YANG Y, Sun Y, Yi W, Li Y, Fan C, Xin Z, et al. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res. 2014;57(4):357-66. doi: 10.1111/jpi.12175.
YU L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, et al. Melatonin receptor mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014; 57(2):228-38.
ZHANG, Y. et al. Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget. 2017.
ATTACHMENT
Figure 1: Evaluation of TBARS levels (nmol / mg protein) in the cardiac tissue of the animals in the different experimental groups) * Means followed by the same letter do not differ significantly by the Kruskal-Wallis test with Dunn’s post-hoc test (p < 0.05). CG: control; GI: induced to hyperlipidemia; GIM: induced to hyperlipidemia and treated with melatonin.
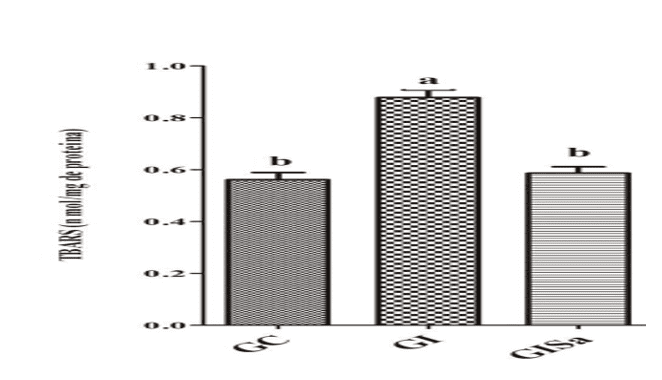
Figure 2. Evaluation of GSH levels (nmol / mg protein) in the cardiac tissue of the animals in the different experimental groups) * Means followed by the same letter do not differ significantly by the Kruskal-Wallis test with Dunn’s post-hoc test (p < 0.05). CG: control; GI: induced to hyperlipidemia; GIM: induced to hyperlipidemia and treated with melatonin.
Table 1: Biochemical analysis of experimental groups
 [1] Graduated in Nutrition from the Uninassau University Center.
[1] Graduated in Nutrition from the Uninassau University Center.
[2] Master’s student in Community Nutrition and Public Health from FCNAUP Graduated in Nutrition from the Uninassau University Center.
[3] Guidance counselor. PhD in Animal Bioscience. Master’s degree in Animal Bioscience. Specialization in Morphology. Degree in Degree in Biological Sciences.
Submitted: August, 2020.
Approved: October, 2020.