ORIGINAL ARTICLE
VILANCULO, Jossias Arnaldo [1], MUTIMUCUIO, Inocente Vasco [2], SILVA, Carlos Santos [3]
VILANCULO, Jossias Arnaldo. MUTIMUCUIO, Inocente Vasco. SILVA, Carlos Santos. Active methodologies for teaching and learning physics: Case study of the formulation of heat and temperature concepts. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 05, Ed. 09, Vol. 07, pp. 84-107. September 2020. ISSN: 2448-0959, Access Link: https://www.nucleodoconhecimento.com.br/education/heat-and-temperature, DOI: 10.32749/nucleodoconhecimento.com.br/education/heat-and-temperature
SUMMARY
The research is part of science teaching, whose objective is to bring teaching approaches that allow an active participation of the student, allowing the students’ alternative conceptions to be valued, enabling the student to build his/her own knowledge. The research is part of David Ausubel’s theoretical assumptions and was developed in a Mozambican school with 9th grade students. The work emerges as a way to bring alternatives to the traditional method of teaching physics predominantly to the whole world. Two classes of 101 students were submitted to a questionnaire elaborated by the researcher in order to identify the alternative conceptions of the students in the concepts of heat and temperature. Then they were submitted to a didactic intervention on the concepts of heat and temperature using the active methodology in the experimental and traditional class in the control class, at the end a post-test was applied. The results show that the strategy used in the experimental class allowed greater participation of students during classes and also noticed a substantial improvement in the formulation of heat and temperature concepts, having significantly reduced alternative conceptions about these concepts.
Keywords: Physics teaching, active methodology, heat, temperature.
1. INTRODUCTION
In 1963, the first Inter-American conference on the teaching of physics was held, in which Richard Feynman, an American physicist and winner of the 1965 Nobel Prize in Physics, was already very pessimistic about the education of physics teaching anywhere (PEREIRA, 2010). Feynman’s concern was in the way physics was taught in schools and the students’ concern about studying only to memorize and reproduce the contents on the day of the test, without bothering to perceive physics.
Although decades have passed since Feynman’s findings to the present day, problems in physics teaching persist. It is not new that in schools worldwide the traditional method is still predominant, where the teacher goes to the classroom concerned with telling the student what he should know and, on the other hand, the student is responsible for writing down and looking for ways to deepen the content addressed. As a result, the student cares only about memorizing, there is no place for him to learn and after a short time forgets everything. The consequence of this is the lack of motivation to learn physics and ends up considering physics as a difficult science, which has nothing to do with its reality.
According to Barroso; Rubini and Siva (2018, p.2) “in the period between the 1970s and 1990s, researchers in physics teaching realized the need to understand what students brought as cultural and conceptual baggage so that they could develop learning processes in Physics”. This movement allowed a significant step in the development of research in the area of science education. It is during this period that some researchers assume the role of previous knowledge or alternative conceptions of the student in learning new concepts, with some assuming that they should be replaced with scientific concepts and others defending their accommodation.
Later, under Vygotsky’s influence, alternative conceptions maintained their importance in learning. Vygotsky’s approach is not to replace alternative conceptions in students, but to take them into account. To this day, the debates have been in this perspective.
Alternative conceptions are the initial ideas presented by the students and that differ from scientific knowledge.
On the origin of alternative conceptions, researchers such as Caldeira and Martins (1990); Thomaz et al. (1994); Mutimucuio (1998); Hülsendeger, Costa and Cury (2006); Araújo and Souza (2015); Leo and Kalhil (2015); Krause and Scheid (2018) converge in affirming that the models that individuals use to explain concepts and physical phenomena of the day-to-day develop from childhood, from the moment the individual comes into contact with nature, others through the common census, not being originated exclusively from their school learning.
Nowadays, the importance of valuing alternative conceptions in the teaching and learning process is agreed. Ausubel, in his work defends the role that the knowledge that the student acquires throughout his experiences interferes in the understanding of new concepts in the school environment.
In order to make his contribution to the theme on physics teaching, the author brings here a study conducted in a Mozambican school in the approach of the concepts of heat and temperature in the classroom with 9th grade students.
According to Faccin and Garcia (2017, p.19), “understanding the concepts of heat and temperature becomes difficult, possibly, because they are also used in everyday life with meaning different from that accepted scientifically.” It is common for someone to pronounce words like “today is hot”, as if the heat were measured from the temperature, but to say today the temperature is high, or “in summer it is very hot and in the winter very cold”, as if the heat and cold were contrary terms, but meaning in summer the temperature is very high and in winter is very low.
From these concepts, heat and temperature, the author brings an approach using an active methodology, where the student is the author of his own knowledge (BACICH and MORAN, 2018).
The active methodologies strengthen the autonomy of students, with them, students are able to build and rebuild their knowledge instead of receiving it passively from the teacher, becoming more questioning being able to intervene consciously and transform reality (PUHL, 2017).
The Author hopes that with this research he will make his contribution to the approach of physics classes using active methodologies, where the student is autonomous and author of the construction of scientific knowledge.
2. EXPERIMENTAL ACTIVITIES AND THE SIGNIFICANT LEARNING THEORY OF AUSUBEL
David Paul Ausubel was an American psychologist who was concerned with studies of learning processes. He developed a theory that became known as Ausubel’s Theory of Significant Learning.
According to Ausubel; Novak and Hanesian (1968); Masini (2011) apud Silva (2020, p.2), “a Teoria da Aprendizagem Significativa (TAS) describes how the individual learns as new knowledge is incorporated into his cognitive structures, based on relevant previous knowledge”.
According to Silva (2020, p.2), “this Theory originated from the dissatisfaction experienced by Ausubel in his schooling, which was characterized by the absence of conditions that contributed to his professional development and to the learning of new knowledge by the other students”.
According to Moreira (2011), Ausubel defines Significant Learning with being:
A process through which new information (new knowledge) is related in a non-arbitrary and substantive (non-literal) way to the cognitive structure of the learner. It is in the course of Significant Learning that the logical meaning of the learning material becomes a psychological meaning for the subject. (MORREIRA, 2011)
For Ausubel (1963) Meaningful learning is the human mechanism, par excellence, to acquire and store the vast amount of ideas and information represented in any field of knowledge.
Ausubel highlights previous knowledge as fundamental for significant learning. According to this psychologist, teaching should focus on what the learner already knows, on their previous cognitive structures (subfunctions), otherwise there will be no Meaningful Learning, but mechanical learning.
From Ausubel’s perspective, the teacher should be concerned rather about knowing what are the students’ previous knowledge or alternative conceptions about the concept to be addressed. This approach can be made possible through experimental activities where they can be problematized.
Experimental activities constitute potentially significant materials. Ausubel refers to potentially significant materials as another essential element for meaningful learning beyond prior knowledge.
From the proposed experimental activities, the teacher elaborates problem questions, where the student when seeking solutions to the questions posed by the teacher, will have the opportunity to expose his alternative conceptions.
Experimental activities are considered singular didactic strategies that contribute to a significant learning in the classroom. Historically since the 1960s, several attempts to improve the quality of science teaching have been based on experimental activities (CATELANI and RINALDI, 2018).
This strategy provides a change of passive attitude for the active, both for the student and for the teacher, because the learner ceases to be a mere observer of the classes and, starts to argue, to think, to act, to interfere and to question (CATELANI and RINALDI, 2018).
According to Pereira (2010), the use of experimental activities as a didactic strategy for the teaching of physics should follow the following steps:
- Proposal of the problem;
- Survey of hypotheses;
- Preparation of the work plan, that is, how the experience should be carried out;
- Assembly of the experimental arrangement and data collection;
- Data analysis;
- Conclusions.
According to the same author, the work plan should contain: objectives, problem, hypotheses and solution of the problem.
3. RESEARCH METHODOLOGY
Here is a description of the methodological approach of the research, the instruments of data collection and the techniques of analysis and interpretation of the results. We worked with two classes, one of control and one experimental, which were submitted to a pre-test before the intervention and then to a post-test after the intervention.
From the point of view of addressing the problem, the research is qualitative and quantitative. According to Gerhaldt and Silvera (2000, p.31-41),” both quantitative and qualitative research present differences with the strong elements of one to complement the weaknesses of the other and vice versa”.
The quantitative approach, in the perspective of Silva and Menezes (2001, p.20) “means translating into numbers opinions and information to classify and analyze them”. In this case, the students’ alternative conceptions will be grouped into percentage frequency tables for their analysis.
It is also qualitative because the explanation and interpretation of the concepts will not require statistical techniques, but rather the natural environment, where the direct source for data collection is the researcher and is the key instrument (SILVA and MENEZES, 2001).
As for the objectives, it is exploratory, because, as Severino (2017, p.91) explains, “it seeks only to raise information about a particular object, thus delimiting a field of work, mapping the conditions of manifestation of this object”.
The study was done through a questionnaire (pre-test and post-test) applied to students at two different times. The objective was to identify the alternative conceptions of the students about the concepts of heat, temperature, direction of spontaneous heat transfer and thermal balance and, from it, identify the control and experimental class.
The instrument before the application was approved by two teachers specialized in the subject, by the way the supervisor and co-supervisor and, read before the application by two physics teachers of the school where the research was developed.
The sample consisted of 101 students from the 9th grade, since these concepts are formally approached for the first time in this class. This sample comes from 2 randomly selected classes in a universe of 9 classes of the daytime period.
The ages of the sample group are between 13 and 17 years. Before being submitted, parents and/or guardians were allowed to be allowed in a meeting led by the class directors. Previously, the researcher requested permission from the school board through a formal letter.
For the analysis of qualitative data, it was decided to create hierarchical categories according to the level of elaboration of each student’s response, except for questions 1 and 5 that asked for the meanings of heat and temperature respectively in the students’ mother tongues, having been analyzed according to the model of table 1, elaborated by the author.
The categorization was inspired by the models used by other researchers in the area of science research (NEVES; CHARRET and CARVALHO, 2017; COVOLAN and SILVA, 2005; MOÇO and SERRANO, 2002). Thus, three categories were created (A, B and C), where category “A” represents the highest level of preparation of responses and category “C” the lowest level of elaboration.
For quantitative data, bar charts were used.
4. RESULTS AND DISCUSSION
4.1 REPORT OF DIDACTIC INTERVENTION
This section describes the environment in which the classes were developed in the experimental class, where we sought to develop an active teaching and learning methodology based on experimental activities. The structure of the experimental guide followed Pereira’s proposal (2010).
This proposal fits into Ausubel’s theory of significant learning, which defends the valorization of the student’s initial knowledge, in this perspective, all activities begin with a problematization, where the student is asked to formulate hypotheses through his previous knowledge, which in this research are called alternative conceptions.
Activity 1: Measure water temperature in three different basins
Objective: To verify that it is not reliable to measure temperature by means of touch.
Problem: How to know if the water is hot or cold?
Formulation of hypotheses by the students: The teacher asked the students to put the possible answers to the question posed. The students’ hypotheses were as follows:
- Groping the bowls from the outside.
- Dipping your hands into each of the bowls.
- Using the thermometer.
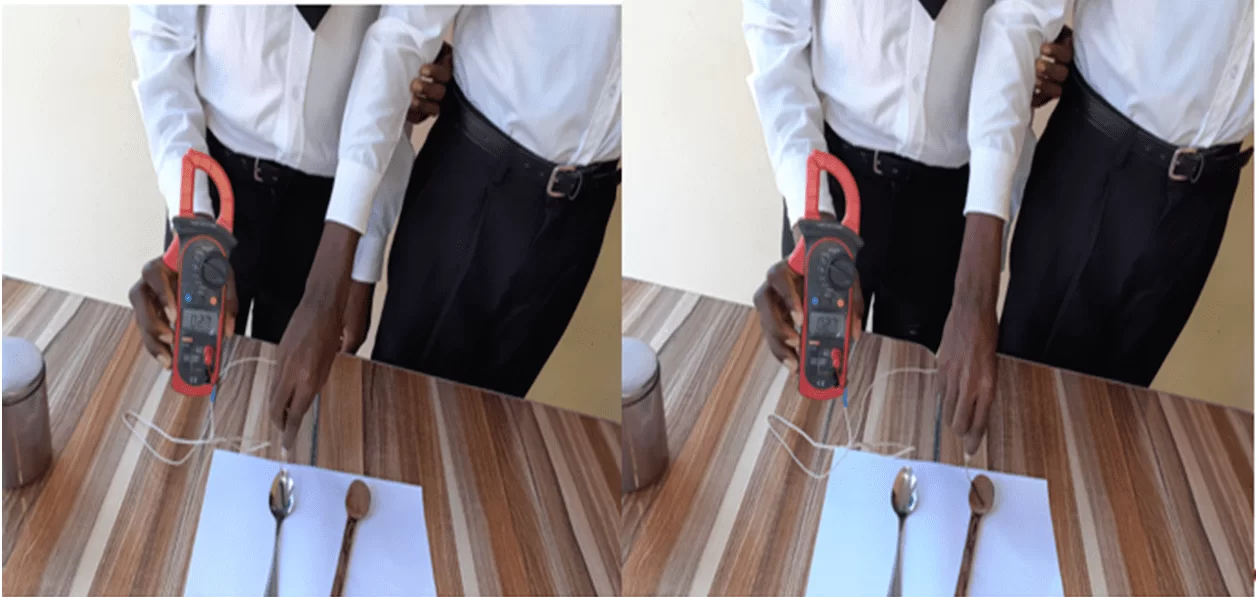
Steps to solve the problem: In this activity two students were asked to dip their hands in a bowl with hot water and another in cold water, see Figure 1. After 30 seconds they removed their hands and plunged into warm water, see Figure 2. You wondered how much you got. The answers were that for the hand that came from hot water, they had the feeling of cold and in the other hand that came from hot water they had a feeling of warmth.
Figure 1: Three bowls with hot, warm and cold water

Source: Author
Figure 2: The moment one of the students dipped both hands in the bowls with hot and cold water, and then the two in warm water.

With these experimental activities, the students came to the conclusion that the sense of touch can give us wrong sensations of temperature, and therefore not recommended for temperature measurement.
Activity 2: Measure the temperature of a body.
Objective: To identify the thermometer as an instrument for temperature measurement.
Problem: We have two almost equal spoons, one of metal and one of wood. How to evaluate the temperature of the two spoons? Compare the two temperatures.
Formulation of hypotheses by students: Students were asked to put some hypotheses after discussion in groups of two to two, resulting in the following:
- Groping the object with the palm of his hand.
- Putting the object in contact with the forehead.
- Measuring your temperature with the thermometer.
- The metal spoon has lower temperature compared to the wooden spoon.
Steps to solve the problem:
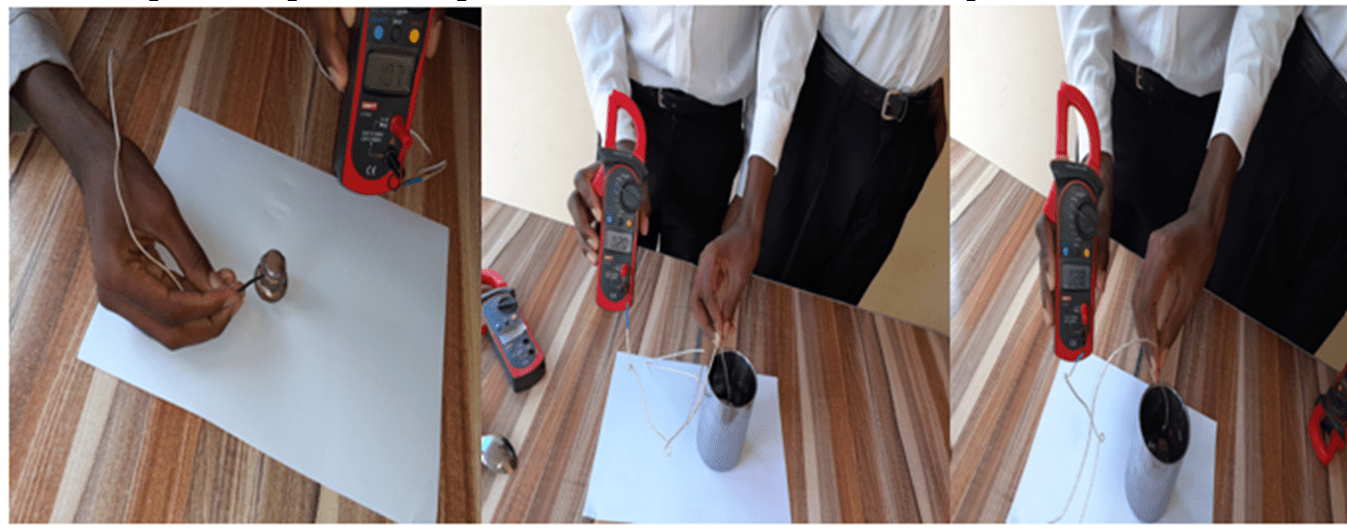
The students were asked to palpate two spoons, one metallic and one wooden, then asked which one had the lowest temperature. Most of them replied that the metal spoon was at a low temperature because it was colder.
After the discussion, the students measured the temperatures of the two spoons using the thermometer (Figure 3) and came to the conclusion that the temperature was the same.
Figure 3: The time of measuring the temperatures of the metal and wood spoons.
Conclusion: it was concluded that the third hypothesis is true, that is, to measure the temperature of a body one must use an instrument called thermometer.
Activity 3: Formulation of the concept of temperature.
Objective: Set temperature.
Problem: What is temperature? What is the relationship between temperature and the degree of agitation of particles?
Formulation of hypotheses by the students: On the question of temperature concept, the students answered the following:
- Temperature is the amount of heat.
- Temperature is internal energy.
- Temperature is the degree of agitation of the particles.
- Temperature is the heating state.
Regarding the relationship between temperature and the degree of agitation of the particles, no student answered.
Steps to solve the problem:
The teacher exhibited three glasses, one with natural water, the other with a mixture of water and ice cubes and finally the third with ice cubes. It asked the students to describe the degree of agitation of the particles with higher, moderate and lower categories and, using the same categories compare the temperatures in the three cups.
The students were able to answer that in the glass with natural water, there is greater agitation of the particles compared to the other glasses and, in turn in the glass containing water and ice cubes, the agitation is greater compared to the glass with ice cubes.
Regarding the temperatures, some students answered that the glass of natural water has a higher temperature, following the mixing glass of ice cubes and water and, finally, with a lower temperature, the glass with ice cubes. However, there is a small number of students who have been undecided on the temperatures of cups 2 and 3, respectively of the mixture ice cubes + water and ice cubes.
To dispel doubts, the teacher asked two students to measure the temperature of the three cups and fill in the table, see Table 1:
Figure 4: The three moments of temperature measurement in the three glasses with natural water, water + ice cubes and ice cubes

Table 1: Experimental data in the formulation of the temperature concept.
| Cups | 1 | 2 | 3 |
| Degree of agitation of particles | Smaller | Moderate | Greater |
| Temperature value | 4º C | 10º C | 25º C |
Source: Author
With this experimental activity the students were able to formulate that the temperature aswells the degree of agitation of the particles.
From the measurement results, it was possible to reach the following conclusion: The higher the temperature, the higher the degree of agitation.
The second class on the concept of heat, had the following sequence of activities:
Activity 4: Formulation of the concept of heat
Objective: Define the concept of heat.
Problem: What is heat? What is the meaning of warmth in your mother tongue?
Formulation of hypotheses by students: In a group of two by two, students were instructed to discuss the concept in Portuguese and then in their mother tongues. The answers given by the students are as follows:
- Heat is the temperature variation.
- Heat means high temperature.
- Heat means energy.
- In the tsuál anguage heat means high temperature.
- In guitonga language heat means high temperature.
- In the tongue chichope heat means high temperature.
About this activity, according to Ausubel (2003), the human being presents the tendency to learn more easily a set of knowledge when he is presented from his most general and inclusive ideas and unfolding to the most specific and less inclusive ideas, that is, from his mother tongue he can present general ideas and, in portuguese, specific ideas.
Steps to solve the problem:
Two students were requested to carry out the experimental activity from the following materials: 4 cups, cold water, and water at room temperature, and thermometer. The others in groups of two students each observed and discussed their observations.
The same amount of water was placed at room temperature in the two glasses (Cup 1 and Cup 2); the initial temperature of the water was measured in the two glasses; 1 cup and cup 2 water was mixed and the final temperature was measured.
Figure 5: The moment of the experimental activity to formulate the concept of heat

Then the same amount of cold water was placed in a glass and in another hot water; (Cup 3 and Cup 4); the initial temperature was measured in the two glasses; the water from cups 3 and 4 was mixed and the temperature of the mixture was measured.
The results were completed in the table below.
Table 2: Experimental data for formulation of the concept of heat
| Containers | T1 | T2 | T3 |
| Cup 1 | 27º C | 55º C | 47º C |
| Cup 2 | 66º C | ||
| Cup 3 | 27º C | 27º C | 27º C |
| Cup 3 | 27º C |
Source: Author
Students were asked to explain the observed phenomenon, answering in groups of 2 students each the following questions: Why in the first mixture (cup 1 and cup 2) the temperature varied? Because in the second mixture (cup 3 and cup 4) there was no temperature variation from T1 to T2? and from T2 to T3?
On the mixtures of cups 3 and 4, almost all students had the same opinion that there was no temperature variation because the two amounts are the same temperature.
In the case of cups 1 and 2, although the students commented that there was a variation in temperature from T1 to T2 because the two quantities were at different temperatures, they differ with regard to the arguments of temperature variation, as we will see below some interventions of students:
Student 1: There was temperature variation because hot water transferred temperature to cold water.
Student 2: There was temperature variation due to the transfer of cold temperature to hot water, hence having lowered to 55º C.
Student 3: Transferred heat to cold water.
Student 4: There was heat transfer from cold water to hot water.
The students were asked what caused the temperature to vary. The researcher explained to the students that the entity that caused the temperature variation was energy (in transit) in the form of heat due to the temperature difference. It has been re-thought that this transfer only occurs when two bodies or substances with different temperatures are put into contact.
The students were asked again, after all what is heat? After several definitions, the students came to the conclusion that heat is the amount of energy in transit due to the difference in temperatures.
Students were asked to examine whether the concept of warmth in mother tongues translates the scientific concept or not, and concluded that in the mother tongues, the concept of heat means high temperature, thus contrasting with the scientific concept of heat.
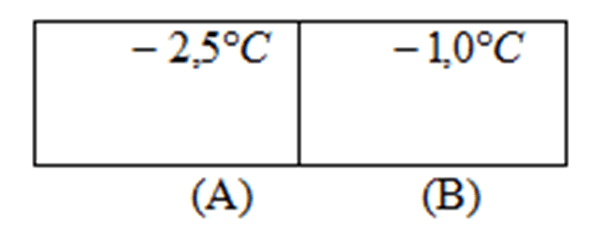
The following questions were asked for group resolution as a form of consolidation: It considers two ice cubes that initially meet at different temperatures as illustrated below. They are put in touch.
Will there be transfer of something between the two blocks? If so, what is transferred between the two blocks of ice? Justify.
The teacher explained to the students that in this case there is also heat transfer because we are putting in contact two bodies with different temperatures, that is, the heat transfer occurs not only for heated bodies but also for cold bodies, as long as they are at different temperatures.
The 3rd class on thermal balance and spontaneous heat transfer direction was developed with the following activities:
Activity 5: Thermal balance and spontaneous heat transfer direction
Objectives: Define thermal balance and identify the direction of spontaneous heat transfer.
Problem: Consider a block (nut of a car) heated to 106º C that is inserted into a glass with water at the initial temperature 20º C. What will be the direction of heat transfer? When does heat transfer stop? The final temperature of the system will be: a. Greater than 106º C; B. 106º C; c. 20º C; d. Greater than 20 C and less than 106º C.
Formulation of hypotheses by students:
The students’ answers were as follows:
- The direction of heat transfer will be water to the block.
- The heat transfer direction will be from the block to the water.
- The heat transfer to when the system is at the same temperature.
- The final temperature of the system will be 20ºC.
- The final temperature of the system will be greater than 106ºC.
- The final temperature of the system will be greater than 20º C and less than 106º C.
Steps to solve the problem:
Using a heater, a block (nut of a car) was heated for 5 minutes, when students answered the questions of the problem. Two students were asked to measure the initial temperature of the heated block, the initial temperature of the water in a glass, and then insert the heated block into the glass with water, see Figure 6. Observe the values read by the thermometer until the temperature stops varying.
Figure 6: The experimental device for the formulation of the concept of thermal equilibrium.
Conclusion: The activity allowed the students to conclude that the direction of spontaneous heat transfer is from the body with higher temperature to the body with lower temperature, this transfer to when the two bodies reach the same temperature, that is, the thermal balance.
4.2 RESULTS OF ALTERNATIVE CONCEPTIONS IN PRE-TEST AND POST-TEST
The categorization of the post-test results is presented in the following tables:
Table 3: The results of the analysis of the students’ answers on the concept of heat, question number 2 (in the pre-test) and question number 1 (in the post-test).
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Pretest | Test | ||
| A | 21 | 72 | Heat means high temperature.
Heat is a substance, a fluid. Heat is contrary to cold. |
| B | 14 | 15 | |
| C | 65 | 13 | |
Source: Author
Table 4: Analysis of students’ responses on the concept of “Turma de controle” (control class – TC) heat
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Pretest | Test | ||
| A | 20 | 22 | Heat means high temperature.
Heat is directly proportional to temperature. Heat is associated only with heated bodies. Heat is a substance, a fluid. Heat is contrary to cold. |
| B | 15 | 7 | |
| C | 75 | 71 | |
Source: Author
Comparing the two tables in the post-test, it is noted that in the experimental class the students had better elaboration of the heat concept, having obtained 72% in category A against 22% of the control class. Although alternative conceptions persist, the experimental class had a lower percentage (13%) compared to the control class (71%). In relation to the analysis before and after the didactic intervention, no two classes noticed a significant improvement in the elaboration of scientific concepts in the experimental class.
The following is presented the result of the analysis of question number 2 through Tables 5 and 6 whose utterance is as follows: “We associate the existence of heat in the following situations: I-A any body in motion, because the whole body in motion has heat; II-Only to hot bodies; III- Situations in which two bodies come into contact, with different temperatures.” This question was only evaluated in the post-test.
Table 5: Analysis of students’ answers to question number 3 of the “Turma experimental (experimental class – TE)” .
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 92 | Heat means high temperature.
Moving bodies have heat. |
|
| B | 3 | ||
| C | 5 | ||
Source: Author
Table 6: Analysis of students’ answers to TC question number 3
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 68 | Heat means high temperature.
Moving bodies have heat. |
|
| B | 12 | ||
| C | 30 | ||
Source: Author
In this question, the difference between the two classes is evident, with the experimental class reaching 92% of concepts with a level of scientific elaboration against 68% of the control class. The level of difficulties or with alternative conceptions is 13% in the experimental class against 30% in the control class.
Tables 7 and 8 show the analysis of the concept of Temperature, question number 4 (in the pre-test) and question number 3 (in the post-test).
Table 7: Analysis of students’ answers about the concept of temperature in TE
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 12 | 89 | Temperature as a measure of heat.
Temperature as something that transfers from one body to another. |
| B | 10 | 6 | |
| C | 78 | 5 | |
Source: Author
Table 8: Analysis of students’ answers about the concept of temperature in TC
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 15 | 82 | Temperature as a measure of heat.
Temperature as something that transfers from one body to another. |
| B | 27 | 15 | |
| C | 58 | 3 | |
Source: Author
Regarding the concept of temperature, the study shows that in both classes there was a significant improvement in the elaboration of the concept. The percentage difference is smaller, being 89% in the experimental class and 82% in the control class. As for the alternative conceptions displayed, the two classes have a lower percentage, however the control class has a lower percentage (2%) in relation to the experimental class (2%). However, this difference is not significant in destiture the method used.
The result of the analysis of question 4.1 (post-test) and 7.1 (in the pre-test) is shown in Tables 9 and 10 below. The question asked of the students was: “You have a metal block initially at 75º C and a calorimeter containing water at 20º C. Then the metal block A is inserted into the calorimeter, according to the following figure:
What is transferred between metal block A and the water in the calorimeter?”
Table 9: Analysis of students’ responses in TE
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 31 | 95 | Temperatura como algo que se transfere de um corpo para o outro. |
| B | 24 | 0 | |
| C | 45 | 5 | |
Source: Author
Table 10: Analysis of students’ responses in TC
| Type | Percentage of students (%) | Alternative conceptions identified | |
| Before | After | ||
| A | 52 | 80 | Temperature as something that transfers from one body to another. |
| B | 35 | 12 | |
| C | 13 | 8 | |
Source: Author
The two tables show that 95% and 80% of students, respectively in the Experimental and Control classes define the concept of temperature. As for the alternative conceptions displayed, only 5% of the students of the experimental class and 8% of the control class also confuse the temperature as something that transfers from one body to another, that is, they confuse temperature with heat. In both classes there were improvements in the formulation of concepts, having reduced the frequency of alternative conceptions, with higher incidence in the experimental class.
4.3 PERFORMANCE RESULT PER CLASS, PRE-TEST AND POST-TEST
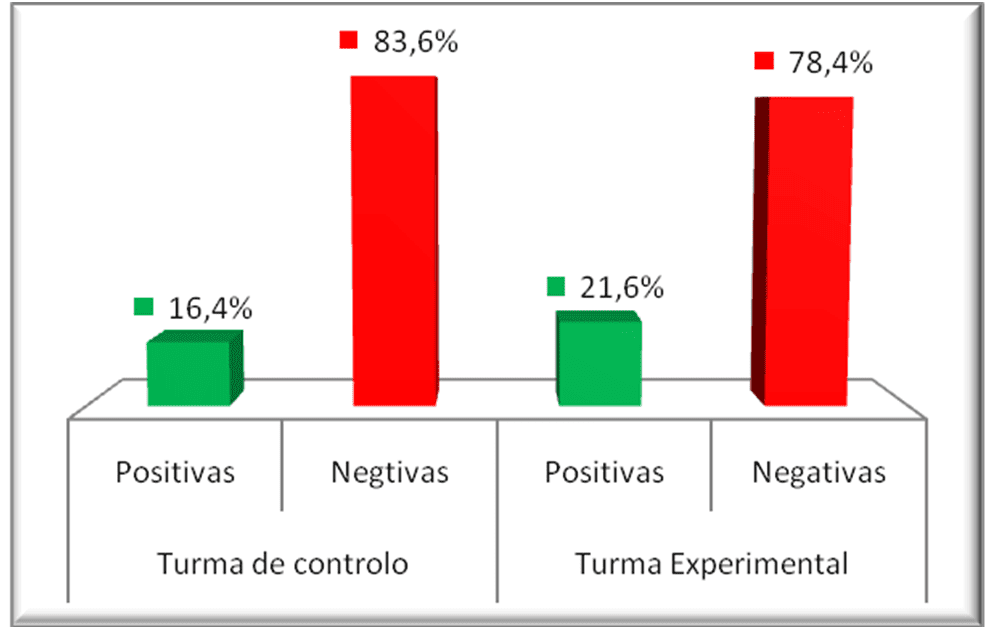
The graphs in Figure 1 show the initial situation of the students before being submitted to didactic intervention in classes 10 (control class) and 11 (experimental class).
Figure 7: Comparative graph of the pre-tests of the experimental and control class
Source: Author
Looking at the chart data although the control class has a slightly higher percentage of positive and negative data than the experimental class, this difference is not significant, as will be demonstrated later. That is, the two classes were at the same level in relation to the contents evaluated.
After the application of the pre-test, the two classes were submitted to a didactic intervention, using different teaching strategies, having been submitted to a post-test. The result is shown in graph 2 below:
Figure 8: Comparative graph of the post-tests of the experimental and control class
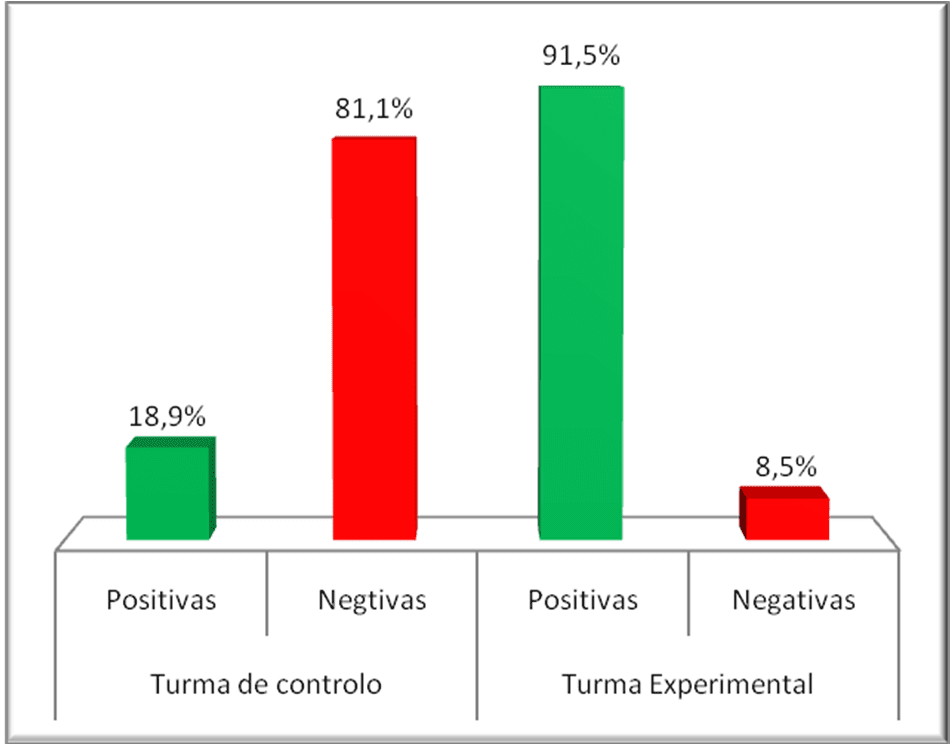
Comparing the two graphs, the difference between the percentage of positives in the post-test in the experimental class is remarkable. Although in the control class there has also been an increase in the percentage of positives, this increase is not substantial. It can be assumed from this evidence that the strategy used contributed significantly to a higher percentage of positive.
5. GENERAL CONSIDERATIONS
The human being, at birth comes into contact with the environment around him. This contact makes there a whole between the human being and nature. It is through this whole that the human being interprets the phenomena of nature before formal contact with the school, hence the student upon arriving in the classroom he already has some previous knowledge.
When introducing new concepts in the classroom, the teacher should have the notion of the existence of alternative conceptions in students and create situations for them to be explored in the classroom so that students have the possibility to differentiate scientific and non-scientific knowledge.
When the alternative conceptions are ignored, there will be no meaningful learning of the concepts.
The research shows that meaningful learning takes place when there is appreciation of the alternative conceptions of students, thus verifying the premise of Ausubel when he says “the most important factor for the learner to assimilate new knowledge is kilo that he knows”.
Alternative conceptions exist in many concepts of physics, and Thermodynamics is the area where students have had many alternative conceptions. Regarding the concepts of heat and temperature, the most common conceptions of students are:
- Heat means high temperature.
- Heat is directly proportional to temperature.
- Heat is associated only with heated bodies.
- Heat is a substance, a fluid.
- Heat is contrary to cold.
- Temperature is synonymous with heat.
- Temperature as a measure of heat.
- The temperature can be transferred,
- Temperature as something that transfers from one body to another.
- Cold bodies don’t contain heat.
- Temperature depends on the nature of the material.
- The direction of spontaneous heat transfer is from the body with mass to body of lower mass.
- In cooled bodies (below zero degree there is no heat transfer
- Temperature is moved by the sense of touch.
- When two bodies at different temperatures come into contact, the final temperature will always be the arithmetic mean temperature of the two bodies.
- The final temperature of two bodies in contact with different starting temperatures will be given by the sum of the two temperatures.
It is concluded that in the mother tongues of the indear students (xitswua, gitonga and cicopi), there is no difference in terms to designate heat and temperature, the most common alternative conceptions of students have as causes of persistence even after formal teaching the following: the language, where the concepts are explained using common sense to simplify their understanding, culture, where the models that the individual uses to explain concepts develop since childhood , not originating from their school learning.
REFERENCES
ARAÚJO, M. De,; SOUZA, P. H. De. Conceitos, Concepções Alternativas E Ensino De Ciência : Uma Investigação Baseada Em Estudos Terminológicos. X Encontro Nacional de Pesquisa em Educação em Ciências, v.1, n.8, 2015.
AUSUBEL, D. P.; NOVAK, J. D.; HANESIAN, H. Educational psychology: a cognitive view. Second Edition. New York. USA: Ed. Holt, Rinehart and Winston, 1978.
AUSUBEL, D.P. Aquisição e retenção de conhecimentos. Lisboa: Plátano Edições Técnicas, 2003.
AUSUBEL, D.P. The psychology of meaningful verbal learning. New York, Grune and Stratton, 1963.
BACICH, L.; MORAN, J. Metodologias ativas para uma educacao inovadora: uma abordagem teorico-prática. São Pailo: Penso, 2018.
CALDEIRA, M. H.; MARTINS, D. R. Calor e Temperatura: Que nocao tem os alunos universitarios destes conceitos? Gazeta Fisica, v.13, n.2, p.85–94, 1990.
CATELAN, S. S., & RINALDI, C. A. Atividade experimental no ensino de ciências naturais: Contribuições e Contrapontos. Revista experiencias em Ensino de Física, v.13, n.1, 2018.
COVOLAN, D. da. & SILVA, S. C. T. A entropia no Ensino Médio: utilizando concepções prévias dos estudantes e aspectos da evolução do conceito. Ciência & Educação (Bauru), v.11, n.1, p.97–117, 2005.
FACCIN, F.; GARCIA, I. K. Proposta de uma unidade de ensino potencialmente significativa sobre temperatura. Aprendizagem Significa em Revista, v.5, n.2, p.18-28, 2017.
GERHALDT, T. E.; SILVEIRA, D. T. Metodos de Pesquisa .Editora, Universidade Federel do Grande Rio Sul, Brasil, 2009.
HULSENDEGER, M. J. V. C.; COSTA, D. K.; CURY, H. N. Identificação de concepções de alunos de ensino médio sobre calor e temperatura. Actas Cientiae, v.8, n.1, p.35–46, 2006.
KRAUSE, J. C.; Scheid, N. M. Concepções alternativas sobre conceitos básicos de física de estudantes ingressantes em curso superior da área tecnológica: um estudo comparativo. Revista Espaço Pedagógico, v.25, n.2, p.227–240, 2018.
LEÃO, N. M. de M.; KALHIL, J. B. Concepções alternativas e os conceitos científicos : uma contribuição para o ensino de ciências. Latin-American Journal of Physics Education, v.9, n.4, p.2–4, 2015.
MOÇO, M. C. C.; SERRRANO, A. S. Analise das Concepcoes alternbativas de estudadntes universitarios de licenciatura em biologia apos uso da internet. IV Encontro Nacional de Pesquisa em Educacao em ciencias, Bauro, 2002.
MOREIRA, M.A. Física de Partículas: uma abordagem conceitual e epistemológica. São Paulo: Editora Livraria da Física, 2011.
MUTIMUCUIO, I. V. Improving Student’s Understanding of Energy: A Study of the Conceptual Development of Mozambican First-Year University Students. Gaza, Moçambique, 1998.
NEVES, J., CHARRET, I.; CARVALHO, S. Estudando a física do efeito estufa no 9o ano: uma abordagem visando a aprendizagem significativa. Experiências Em Ensino de Ciências, n.9, 2017.
PEREIRA, M. M. Uma proposta para o ensino de calor e temperatura no ensino médio. Dissertação (Mestrado em Ensino de Física). Instituto de Física, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 2010.
PUHL, N. M. Atividades Investigativas No Estudo da Termodinâmica: Incentivando a Autonomia do Estudante. Dissertação (Mestrado em Ensino de Ciências Exatas. Universidade do Vale do Taquari, Lajeado, 2017.
SEVERINO, A. J. Metodologia do trabalho científico. 23.ed. São Paulo: Cortez, 2007.
SILVA, E. L. & MENEZES, E. M. Metodologia da pesquisa e elaboração de dissertação. 4ª ed. Florianópolis, 2001.
SILVA da, J. B. A teoria da Aprendizagem Significativa de David Ausubel: uma análise das condições necessárias. Research, Society and Development, v.9, n.4, mar., 2020.
THOMAZ, M. F., MALAQUIAS, I. M., VALENTE, M. O.; ANTUNES, M. J. Uma alternativa para ultrapassar concepções alternativas sobre calor e temperatura. Gazeta de Física, v.17, 1994.
[1] Master’s degree in Education / Physics Teaching. Graduated in Mathematics and Physics Teaching.
[2] Advisor. Professor Doctor.
[3] Co-advisor. Professor Doctor.
Sent: July, 2020.
Approved: September, 2020.