RODRIGUES, Rodrigo Vieira
RODRIGUES, Rodrigo Vieira. Naphthenic acids: detection and identification in petroleum. Multidisciplinary Core scientific journal of knowledge. 03 year, Ed. 06, vol. 07, pp. 81-90, June 2018. ISSN:2448-0959
Summary
The natftênicos acids (AN) represent a very small content in crude oils, as in derivatives, of which they are components. The very low concentration of these acids, in arrays with wide and varied composition, hinders your extraction. Therefore, the separation, concentration and purification of the sample, in addition to the analysis, require time and effort. The aim of this study was to analyze how accomplished the detection and identification of naphthenic acid in petroleum and their final applications. A descriptive bibliographic characteristic research on the subject. The study showed that there is no validated extraction method which makes it possible to extract the oil in sufficient quantity to structural identification. Some techniques such as open column chromatography and high performance liquid chromatography (HPLC) are widely used as analytical techniques for the acid fractions, nuclear magnetic resonance spectroscopy of hydrogen and carbon (NMR-H and NMR-C), Fourier transform infrared (FT-IR) 77.78 and mass spectrometry (MS). However it was concluded that the solid phase extraction in a non-aqueous, employing anions exchanger adsorbent, is a selective and relatively agile, efficient extraction and isolation of naphthenic acids from crude oil.
Key words: Naphthenic Acids, extraction, oil.
1. Introduction
In recent years much of the oil reserves discovered in Brazil, this is heavy oil of low level API (< 20th), high viscosity and high total acidity (FORTUNY, et al, 2008).
The results of the findings indicate that crude oil sources in South America, are among the compounds with higher acid value in the world. So this gives the oil acidity a peculiar feature that leads to an often inappropriate use in the use of this raw material for refining and reuse of waste (JACQUES, et al, 2012).
The naphthenic acids (AN) are identified in immature oils, are biodegradable, in heavy oils and waters with waste generated in bitumen extraction process (GRUBER et al., 2012). Is an acid that is important in the field of geochemistry, on trade, environment and corrosion phenomena (WU et al., 2004).
According to Qian et al. (2001) chemically naphthenic acids are carboxylic acids present in petroleum, form a fraction consisting of thousands of different compounds, being more common with monocarboxylic acids the carboxyl linked to a chain alicíclica containing one or more twin cycloalkanes.
Thus the term means when this compound is naphthenic found through saturated cyclic compounds, that with your use classifies all types of acids detected in petroleum, even the linear and branched saturated. So these types of carbo xílicos acids found in oil show up as a serious difficulty for the effectuation of the refining and cracking, due to corrosion feature in your lines of mass transfer and heat, in the sections of entry and reflow of columns (at atmospheric pressure and vacuum) and capacitors of the refinery distillation units, and with this characteristic of the scholars perform often analysis as it pertains to your structure in the sense of being tapped so as not to disturb the oil (EDGE, et al, 2008).
In order for this acid can be analyzed in the period in which occurs the extraction of oil is used a technique that evaluates the content by titration with KOH, originating the naphthenic acid number or index. This index is used to analyze the content of the sample, but corrosion does not interfere with the quality of the oil and the nature of acids present (fields, 2005).
So, to characterize these acids, it is necessary to extract them and analyze them individually in order to assess the level of corrosion. Therefore, this study aimed to analyze how detection and identification is performed of naphthenic acid in petroleum and their final applications.
2. Naphthenic acids
Acid-related studies dating back more than a century and these are present in a variety of sources of hydrocarbons such as crude oils and oil sands. In 1883 Markownikoff acknowledged the presence of acids present in crude oils from Azerbaijan containing the carboxylic group associated with carboxylic acids. Since then, the term naphthenic acids has been used to explain all the acidic species containing the carboxylic group in crude oils (CAMPBELL, 2005).
2.1 properties of naphthenic acids
Currently, the definition of the American Petroleum Institute of naphthenic acids includes structures with cyclopentane and cyclohexane rings fused with multiple structures or simple with a carboxylic group attached to an aliphatic side chain or ring cicloalifático (American Petroleum Institute, 2003). It became a common practice to represent the naphthenic acids in crude oil by the following general formula:
CnH2n + ZO2 (01)
Where n is the number of carbon and Z is the deficiency of hydrogen. Z values can be 0 or a negative integer, where the Z value reflects the loss of a hydrogen atom that occurs when a cicloalifático ring is present in acidic structure. Thus, Z = -2 is equivalent to an acid with a ring (mono-cyclical), Z = -4 is equivalent to an acid with two rings (bi-cyclic). More than one isomer normally exists for a given Z value and that makes the correct species identification of naphthenic acids, however, usually the naphthenic acids from crude oil, on average, have molecular weights between 200 and 700 Daltons (OLIVEIRA et Al, 2004).
The naphthenic acids have different characteristics when examined, and may vary between the dark tint and when in distillation process go on to have more color to light yellow and may go to Amber hue. They also have a feature as the texture being classified according to your high viscosity and often to be differentiated from other compounds is performed an analysis on refraction and density. The average molecular weight naphthenic acids derived from diesel oil with Group-COOH connected directly to the ring is 260, and for those with n ≥ 4 is 243, those with higher molecular capacity are extracted from fractions of oil refining with ebuliç point more high (fields, 2005; OLIVEIRA et al., 2004).
Whereupon the naphthenic acids and their salts present in significant amount in water and oil, favoring thus the composition of colloidal solutions. But what must be assessed is the emulsion created through water and oil lead to changes and complications in input and output systems of tabs for evaluation of compounds. So the phenomenon that occurs is the decomposition of poorly soluble metal salts in water, such as Ca and Mg, causing nucleation, generating an agglomeration of particles, which are responsible for frequent operational interruptions in the processes of separation (ST JOHN et Al, 1998).
2.2 applicability of naphthenic acids
Naphthenic acids are found in Commerce in General in terms of chemical preparations and also in technical mixtures, because these preparations are used as criteria for quality indicators and evaluation of the presence of AN in order to characterize samples in industry and in research, because with that assessment may be technical certifications (CAMPBELL, 2005).
Thus a mixture with naphthenic acids can be prepared through the use of crude oil or fractions since commercial acid is found in distillates such as kerosene and diesel. There are several extraction processes for commercial purposes, but the most common is the extraction with caustic soda, however it is possible to quote also distillation, extraction and ion exchange extraction with solvents (OLIVEIRA et al., 2004).
In this way a commercial preparation regarding the purity of your mixture is related to the method of refining and oil used in the source which was extracted. The commercial may have components in your composition phenolic compounds and sulphur compounds, fatty acids and aromatic, but often in preparations carried out through the same process and array can present significant differences (VAZ, 2005).
Before the exposed to large-scale industry uses this compound and the various mixtures which derive, such as the metallic salts of these acids, like the naphthenates (metal salts of naphthenic acids) that are used to ensure the manufacture of Paint driers, corrosion inhibitors, catalytic organic reactions and lubricants (VAZ, 2005, OLIVEIRA et al., 2004).
The can also be used for copper extraction together with the predominant way naphthenates will act for use in environmental preservation with regard to wood is also used as emulsifying agents in the production of insecticides (GRUBER et al., 2012).
The quantification of acidity found in oils is targeting the bacteria that promote through your degradation increased acidity and can be identified in oil reservoirs where through the biodegradation of oil can be manipulated and identified as the carboxylic acids. Given this, and the acidity of these medium molecular weight acids (C10-C20) which were quickly produced, Watson et al. (2002) found that the production of this material was also present in samples of n-alkanes. Also assessed this particularity of Meredith et al (2000) which identified the influence of carboxylic acids in the acidification process of crude oils. In this study the authors compare values of acidity and acid fraction analysis of 33 samples of different oils and the result showed that biodegradation is a preponderant factor for which the carboxylic acids in high concentration in petroleum (GRUBER, et al, 2012).
With regard to polar compounds which are important quantities in oils the results with biomarkers, saturated and aromatic fractions have been the subject of much of the geochemical studies, resulting in analyses that identify the source and maturation of the oil. Thus, Nascimento et al. (1999) and Galimberti et al. (2000) discussed in his studies the use of polar compounds, among them the naphthenic acids as biomarkers, being these important substances with properties and information essential to assess interactions of water, oil and rock in order to arrive at an analysis of the origin and preservation of these compounds.
The AN are widely used in environmental disasters as in the case of fuel oil spill and can still have applicability in fingerprint analysis, do support more than the non-polar alkane hydrocarbons, isoalkanes and aquilcicloexano and this can be used in the identification of the origin of the oil that caused the crash (GRUBER et al., 2012).
2.3 damage caused by naphthenic acids
The naphthenic acids can cause several damage from your composition primarily with regard to corrosion and this fact was primarily described in refining equipment in the year 1920 as these indicate AN acids are mainly responsible corrosion of materials in liquid phase at the time of refining. This type of corrosion has not yet been studied and explained in an ideal context to such suffering the influence of several factors. The factors influencing corrosion studied are the type of acid, corrosive compounds of crude oils characteristics and the way your processing occurs as your temperature among other factors. The studies show that corrosion occurs through and a mechanism of metal Chelation by carboxylate anion with the formation of hydrogen gas (ZANIN, et al., 2002).
Corrosion caused by naphthenic acids occurs when used at temperatures ranging between 220 and 400° C, concomitant to that on corrosion which grows with oil is also the increase in concentration of AN, but what if evaluated in the studies was that the size and the rate of corrosion is targeting the carboxyl group which leads the complex metallic and composite type. Thus it is important that the distribution of the ring type and number of carbons since the corrosiveness is linked to the size and structure of AN (ZANIN, et al., 2002).
According to Zanin et al (2002) it is difficult to analyze AN about your presence in conventional crude oils, this is due to the fact that if has shortage of jobs that evaluate that substance in refineries and in the environment, due to the difficulties for an analysis correct and needs of these compounds. Wong et al. (2009) analyzed this compound by using an activated carbon unit used as a guide to assess effluent pretreated to a refinery. The AN that these compounds were found confirmed the presence of these acids in the final waste water of refining, but this treatment also helped in the reduction in the aquatic environment.
The use of AN industry and its compounds represent the appearance of these compounds in the environment, through the disposal of refinery waste water as well as in environmental disaster which has fuel oil spills. This is due to the fact that AN own water solubility reporting as well as naphthenates, in neutral or alkaline pH with the mobility characteristic in oil-contaminated surface waters (WONG, et al, 2009).
2.4 Oil
Oil had your origin from the appearance of source rocks, these emerged from the formation in the production, accumulation and preservation of a given fraction of organic matter. The first matter that originates from oil is synthesized by living organisms, secondary to this process and that matter if segments originating the petroleum (GALLARDO, 2009).
Oil generation occurs from the evolution of organic matter with its main stages of development of organic matter; Diagenesis, catagenesis and metagênese, these stages are present in all types of sediment. But the most important factor that identifies the process of generation of oil is the source of organic matter, temperature and time. Therefore the quantity of hydrocarbons present, the composition and the depth of oil and gas generation are the most important as they may vary (GALLARDO, 2009).
On the exposed oil in refined form, has the nomenclature of raw oil and your composition may be influenced by several factors and mainly in his compositions as migration, biodegradações and chemical transformations. In this way the resulting oil is a composite of various proportions resulting in composite oils and different characteristics, such as color, viscosity, density, acidity and sulphur content (CAMPBELL, 2005).
2.5 oil Composition
According to Campos (2005) the components of petroleum and its fractions can be classified in: hydrocarbons and heterocompostos. Hydrocarbons are organic compounds of carbon and hydrogen, and stand out for being the most common compound found in oil with concentrations ranging from 50 to 90% in weight and are divided into:
- Alkanes or paraffins are characterized by having a normal chain or branched, also regarded the normal alkanes and isoalkanes.
- Cyclic alkanes, naphthenes, are in your composition one, two or more saturated rings being so classified as mono, di and polycyclic musks. Its structure has five or six carbon atoms but may present with one or more branches alquílicas in the ring.
- Alkenes or olefins are characterized with structures that can be branched or cyclic, normal, however the olefins are not present in crude oils and their derivatives.
- Aromatic, have on average a benzene ring and may have alquílicas and naftênicas branches. Are sorted from the number of rings present in the structure, which receive the name of mono, di or polyaromatic those containing one, two or more aromatic rings.
According to Oliveira et al. (2004) hydrocarbons are a group very analyzed in geochemical and environmental studies, especially the normal alkanes and isoalkanes, naphthenes and aromatic, and can also be classified into molecular fossils or Biomarkers. These compounds are widely used for reporting deep-water depositional environments and in these cases indicate the presence of contamination and your source in water and sediment, degree of maturation of source rocks and organic matter degradation level .
According to Zanin et al (2004) the heterocompostos are the least found featuring a total of up to 15% in weight of the crude oil in your composition is part of the carbon and hydrogen, which give rise to the heteroatom if sorting according to your presence :
- Sulfur compounds: aliphatic, aromatic and are, one can find sulfur in elementary form.
- Nitrogen compounds: present at concentrations of 0.2 to 2% and on average 90% of the crude oils have a content of less than 0.2% by weight of nitrogen. If subdivided in neutral, basic nitrogen compounds and acids.
- Oxygenated compounds: the oxygen that are in most raw oils are low and are around 0.1 to 1.5%. Carboxylic acids present in various compounds and among them there are the phenols, naphthenic acids, furans and fenilcetonas.
These compounds also present themselves in the form of salts or their respective acids, such as:
- Compounds containing metals: oil contains a variety of metals, gaining the vanadium, nickel and iron.
- Resins and asphaltenes: are complex that have high C/H, also comprise of sulfur, nitrogen and oxygen. Asphaltenes are studied as the fraction of the oil that is insoluble in heptane or pentane, remaining in colloidal form in the oil. The resins, do the opposite effect to that of asphaltenes and are readily soluble in the same solvents. Vanadium and nickel porphyrins are part of asphaltenes. The molecular structures of asphaltenes and resins are not well known due to the chemical complexity.
According to Campos (2005) in the use of oil fractions, products and components are often used through the manner in which they were obtained or by operational procedures involved. There are comparative data between light fractions and high aromatic content that get heavier if compared on the basis of paraffin. Given that the heavy fractions are determined, because it takes an action through enrichment of polar compounds, such compounds can also be described as oil impurities, with molecules of aromaticity and variable different heteroatoms and functional groups.
2.6 identification of naphthenic acid in petroleum
The components that make up the oil acids were characterized by the year 1955 and, in this work, we identified two acids with up to ten carbon atoms, except for the fatty acids (OLIVEIRA et al., 2004).
According to Oliveira et al. (2004) other studies have shown that in several crude oil samples collected around the world who had until 5% of naphthenic acids. These studies also determined that concentrations and compositions of naphthenic acids are linked the origin of the oil, so the low presence of naphthenic acid in petroleum makes it necessary to use pre-merger steps or more extractions to remove sufficient amounts for future analysis. Currently, there is a method to identify or quantify individual acids, and the analytical methods used deal with such acids as a group or sub-groups based on distribution of n and Z (number of carbons and rings).
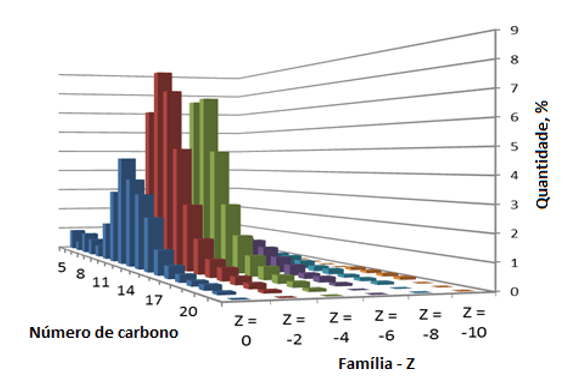
The work published by Rogers et al. (2003) reports tests from extracts of oil sands, which was composed of a higher proportion of isomers of naphthenic acids of higher molecular weight, where three and four ring constituents understood 38 % of the test specimen. Figure 1 presents the distribution of n and Z.
According to Campos (2005) and Seifert et al. (1969) the extraction with alkaline solutions, open column chromatography, high performance liquid chromatography with silica modified ion exchange and chromatography are techniques widely used to achieve oil and acid fractions is important the use of separation steps and sufficient quantities of solvents in order to work and complex solutions of polar compounds. Is also quite described in studies that the withdrawal of acid extracts by liquid-liquid using alkaline solutions and ammonia is a widely used method. The authors Seifert and Teeter (1970), Seifert et al. (1969), Dzidic et al. (1988) and Wang et al. (2006) record exhaustive extraction schemes and with great volume of solvents can cause a deficiency in acids isolation due to the formation of emulsions and co-extração acidic impurities such as phenols and carbazóis.
2.7 techniques for analysis of naphthenic acids
Santestevan et al. (2008) describes the petrochemical companies employ analyses for the acid fractions, nuclear magnetic resonance spectroscopy of hydrogen and carbon (H-NMR and NMR-C), Fourier transform infrared (FT-IR) and mass spectrometry (MS).
Studies of Tomczyk et al (2001) identify 40% acid compounds with the use of liquid-liquid technique, of these only 10% had two oxygen atoms in the structure identified as typical carboxylic acids and the result of this study it became clear the acidic components present in the oil as a result of biodegradation by microorganisms.
The technique of mass spectrometry in accordance with Qian et al. (2001) used in masses for assessing acidic fraction obtained by solid-phase extraction (SPE) of crude oils has determined the presence of acids with n varying from 15 to 55 and Z between -2 and -12 (a six anéi naphthenic) and s the presence of aromatic acids with up to 3 rings and structures containing more than two oxygen atoms. Before it is validated the degree of complexity of the acidic fraction of crude oils and indicate the prevalence of phenols and other carboxylic acids.
Given that according to Gruber et al. (2009) was evidenced that the technique of gas chromatography coupled to mass spectrometry (GC/MS) is the most appropriate method for providing as much information to identify the structures of inorganic acids naphthenes. But the technique presents a disadvantage, because the electronic impact ionization (EI) leads to excessive fragmentation of the compounds, generating complex spectra that provide limited information about structure and molecular weight of these acids.
That way St. John et al (1998) studied and developed a simple technique for derivatizar AN in esters (tert-butildimetilsilil or t-BDMS) to analyze them by GC/MS with EI ionization mass spectra obtained with a simpler interpretation where the t-BDMS esters are obtained by reaction of derivatizante N-methyl-N-(t-butildimetilsilil) trifluoracetamida, short for MTBDSTFA, with the hydrogen of AN acid.
Conclusions
In recent years the oil discovered comes presented in your composed a more acidic structure causing difficulties for refining and so your for your characterization with regard to marketing, which throughout the ages comes demanding ever more quality and clarity in the description of this compound. The view that there is currently a requirement to identify the nature of acids that lead to problems in the oil and how to best take advantage of them.
This was evidenced in the study to isolate the naphthenic acids and perform an analysis of corrosion or your concentration in oil a technique which presented satisfactory result was the solid phase in a non-aqueous medium, using together a adsorbent anions exchanger. This technique proved to be effective, selective and rapid for naphthenic acids of oil can be recovered in oil and concomitant to this practice the studies also showed that this result analysis for infrared spectroscopy allows determine the number of naphthenic acid (NAN) of oil in comparison to the number of total acidity (NAT), but without the risk of interference from other compounds.
So it can be seen that the primary applicability of naphthenic acids in oil identification is the monitoring of levels of acidity of same that will qualify the acid value of oil and thus determine your degree of corrosion for the industry It is important for your oil applicability and market expansion.
Bibliographical references
Fields, M.C.V. study of naphthenic acids from diesel petroleum-derived heavy Marlin. PhD thesis presented at Universidade Federal do Rio Grande do Sul. Institute of chemistry. Graduate program in materials science. Porto Alegre. 2005.
CLEMENT, J. S.; MACKINNON, M. D.; FEDORAK, P. M.; Envir. Sci. Technol. 2004, 38, 1009.
DZIDIC, I.; SOMERVILLE, C.; LANE, J. C.; HART, H. V.; Anally. Chem. 1988, 60, 1323.
FORTUNY, M.; RAMOS, L. D.; DARIVA, C.; EGUES, S. M. S.; SANTOS, A. F.; Qiu. New. 2008, 31, 1553.
GALIMBERTI, R.; GHISELLI, C.; CHIARAMONTE, M. A. Org. Geochem. 2000, 31, 1375.
GALLARDO, M.T. Tésis of mastery. Simón Bolívar University. (2009) Caracas, Venezuela.
GRUBER, L. D. A., DALLI, F. C., CARAMÃO, E. B., JACQUES, R. A. Naphthenic acids in the oil. Qiu. New, vol. 35, no. 7, 1423-1433, 2012.
GRUBER, L. D. A.; MORAES, M. S. A.; SANTESTEVAN, V. A.; GELLER, A. M.; BORTOLUZZI, J. H.; DALLI, F. C.; MACHADO, M. E.; GARCIA, A. O.; GHOSH, R. C. L.; ZINI, C. A.; CARAMÃO, e. b. characterization of Naphthenic Acids in Heavy petroleum Fractions via Two-dimensional Chromatography. In: XII Colacro: Latin American Congress of chromatography and Related Techniques, 2008, Florianopolis. Book of abstracts, 2008.
MEREDITH, W.; KELLAND, N. J.; JONES, D. M.; Org. Geochem. 2000, 31, 1059.
BIRTH, L. R.; REBOUÇAS, L. M. C. KOIKE, L.; REIS, F. DE A. M.; SOLDAN, A. L.; CHAO, J. R.; MARSAIOLI, A. J. Org. Geochem. 30, 1999, 1175.
OLIVEIRA, c., Eniz ZINI, Claudia Alcaraz, VALE, Maria Mridul, RODRIGUES, Maria Regina, CAMPOS, Maria Cecilia Valenzuela; CARAMÃO, Elina Bastos. Extraction of Nitrogen Compounds from Heavy Gas Oil using Liquid Chromatography and Preparative Three Different Stationary Phases. X COLACRO, 2004, Campos do Jordão/SP.
QIAN, k., ROBBINS, w. k., HUGUEY, c. a., COOPER, h. j., RODGERS, r. p., MARSHALL, a. g. Resolution and identification of elemental with-positions for more than 3000 acids in heavy crude petroleum by negative-ion microelectros-pray high field Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels v. 15, n. 6, p. 1505-1511, 2001.
Rogers v. Mammalian toxicity of naphthenic acids derived from the Athabasca oil sands. Ph.d. thesis. University of Saskatchewan, Saskatoon, Canada, 2003.
SANTESTEVAN, V. A.; GRUBER, L. D. A.; MORAES, M. S. A.; GELLER, A. M.; BORTOLUZZI, J. H.; DALLI, F. C.; MACHADO, M. E.; GARCIA, A. O.; GHOSH, R. C. L.; ZINI, C. A.; CARAMÃO, and b. development of methodology for the fractionation of Heavy Brazilian oil Samples via HPLC for characterization of Naphthenic Acids by gas chromatography. In: XII Colacro: Latin American Congress of chromatography and Related Techniques, 2008, Florianopolis. Book of abstracts, 2008.
SEIFERT, W. K.; TEETER, R. M.; Anally. Chem. 1970, 42, 180.
SEIFERT, W. K.; TEETER, R. M.; HOWELLS, W. G.; CANTOW, M. J. R.; Anally. Chem. 1969, 41, 554.
S JOHN, W. P.; RIGHANI, J.; GREEN, S. A.; MCGINNIS, G. D.; J. Significant Action 6. The 1998, 807, 241.
TOMCZYK, N. A.; WINANS, R. E.; SHINN, J. H.; ROBINSON, R. C. Energy Fuels. 2001, 15, 1498.
WANG, Y.; CHU, Z.; QIU B.; LIU, C.; ZHANG, Y.; Fuel 2006, 85, 2489.
WATSON, J. S.; JONES, D. M.; SWANNELL, R. P. J.; VAN DUIN, A. C. T.; Org. Geochem. 2002, 33, 1153.
WONG, D. C. L.; VAN COMPERNOLLE, R.; NOWLIN, J. G.; O'NEAL, D. L.; JOHNSON, G. M.; Chemosphere 1996, 32, 1669.
ZANIN, Ramakrishna Daiane, SCHUTZ, Priscilla, CAMPOS, Maria Cecilia Valenzuela, CARAMAO, Elina Bastos, extraction of carboxylic acids with Petróleo Brasileiro In: XIV Salão de Iniciação Científica of UFRGS, Porto Alegre, 2002. Book of abstracts of the 13TH CENTURY Hall of UFRGS de Iniciação Científica, 2002.