ORIGINAL ARTICLE
SOARES, Karla Danielle Almeida [1], ALVES, Elizabeth Simões do Amaral [2], SILVA, João Manoel da [3], VIANA, Cibeli [4], CAVICCHIOLI, Valéria Quintana [5], ANDRADE, Andrezza Cavalcanti de [6], ALVES, Aglair Cardoso [7], TORRES, Alisson Rogério dos Santos [8], MOURA, Vilton Edson Figueiroa de [9], SOARES, Anísio Francisco [10], SILVEIRA, Ana Virgínia Marinho [11], MEDEIROS, Elizabeth Sampaio de [12]
SOARES, Karla Danielle Almeida. et al. Antibiotic residues: fresh milk from expansion tanks in Alagoas. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 08, Ed. 11, Vol. 02, pp. 166-173. November 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/agronomy-en/antibiotic-residues, DOI: 10.32749/nucleodoconhecimento.com.br/agronomy-en/antibiotic-residues
ABSTRACT
Milk is a food widely consumed by the population, so it is important that it is produced under appropriate hygienic conditions and free from chemical contaminants of various origins. The objective of this study was to test for antibiotic residues in fresh milk from municipal expansion tanks in the State of Alagoas. 42 milk samples were collected in sterile bottles of approximately 50 mL. These samples were frozen and sent to be subjected to the official analysis methods of the Ministry of Agriculture. In the laboratory, qualitative analysis of multi-residues in different matrices and determination of beta-lactam antibiotic residues in bovine milk by liquid chromatography coupled to mass spectrometry (LC-MS/MS) were performed. In 95.2% (40/42) of the samples analyzed, the presence of antibiotic residues was not detected; however, in two samples (4.8%), the presence of residues was detected, but at levels that could not be quantified. Samples were within the limits established by Brazilian legislation. This study concluded that there were no antibiotic residues at quantifiable levels in the samples analyzed. This study concludes that there were no antibiotic residues at quantifiable levels in the samples analyzed. It is therefore suggested that these antimicrobials be monitored in milk to ensure the absence of these residues and to guarantee a quality product, which is essential for public health.
Keywords: Public health, Food safety, Anti-infective agents, Foods of animal origin.
1. INTRODUCTION
The presence of antimicrobial residues in milk represents a potential risk to public health and one of the main concerns is to guarantee the safety of the raw material, since the presence of chemical substances alters the quality and causes economic damage to the dairy industrial chain, compromising commercial relations and endangering the health of consumers in the microbiological, immunopathological and toxic-pharmacological spheres (Rosa et al., 2023).
This assessment of antibiotic residues is based on the indiscriminate use of veterinary drugs to treat mastitis in dairy cattle, where in most cases the grace period is not respected, resulting in residues of these drugs in animal products, contributing to and promoting the selection of super-resistant bacteria (Brown, 2020).
Brazilian legislation requires regular measurement of the tanks of each vehicle transporting milk to the processing industry, with at least two classes of antibiotics being tested, in compliance with the Maximum Residue Limits (MRLs) for each specific group (Brasil, 2018).
It is worth noting that drugs used to treat animals that are harmful to health must be measured and a MRLs established, which gives a tolerance limit for their presence in food without causing harm to humans or animals (Quintanilla, 2021).
Therefore, it is important to note that beta-lactams are antimicrobials that are widely used in animals with bovine mastitis. However, some bacteria that cause pathology in animals are capable of inducing resistance mechanisms after exposure to these antimicrobials, which affects the effectiveness of treatments, such as the production of beta-lactamase, which is the most common resistance mechanism found in Staphylococcus spp. (El Behiry et al., 2012).
High rates of residue contamination can occur when there is a lack of control over the sale and use of veterinary antimicrobials, awareness among farmers, and improvements in hygiene conditions and the effectiveness of food inspection. (Silva et al., 2023). Considering the public health and industrial importance of the presence of antimicrobial residues in milk, the objective of this study was to investigate antibiotic residues in fresh milk from municipal expansion tanks in the State of Alagoas.
2. MATERIAL AND METHODS
42 samples of fresh milk were collected from community expansion tanks belonging to a Milk Producers’ Cooperative in the state of Alagoas. 100 producers who supplied milk to these community tanks were interviewed. The study was carried out in 23 municipalities belonging to the three mesoregions of the state, which have different physical, economic, social and cultural characteristics (Agreste Alagoano, Leste Alagoano and Sertão Alagoano). The samples were collected in sterile vials of approximately 50 mL, frozen and sent to Laboratório Nacional Agropecuário do Rio Grande do Sul to be subjected to the official methods of the Ministry of Agriculture. In the laboratory, qualitative multi-residue analysis was performed on various matrices and residues of beta-lactam antibiotics were determined in bovine milk using liquid chromatography coupled to mass spectrometry (LC-MS/MS).
- For the Multiresidue analysis, 500µL of milk was used and the protocol recommended by the Ministry of Agriculture, Livestock and Supply (MAPA) was followed. Positive controls fortified at the MRL and CCβ (spiking levels at which the procedures of this method have a false negative rate of less than 5%) and three samples fortified after the extraction process were used. Milk samples were then extracted and controls prepared, followed by detection of residues in the chromatograph. The results are expressed as positive and negative for the presence of residues. Positive results were confirmed using the LC-MS/MS method to quantify the levels detected. The analytes studied belong to the classes: Tetracyclines (Tetracycline, Oxytetracycline, Chlortetracycline, Doxycycline); Sulfonamides (Sulfadiazine, Sulfatiazole, Sulfamethazine, Sulfamethoxazole, Sulfaquinoxaline, Sulfadimethoxine, Sulfadoxine, Sulfachlorpyridazine, Sulfamerazine, Sulfizoxazole); Quinolones (nalidixic acid, oxolinic acid, flumequine); Fluoroquinolones (ciprofloxacin, enrofloxacin, difloxacin, sarafloxacin, danofloxacin, norfloxacin).
To determination of beta-lactam antibiotic residues in bovine milk, 2 mL of milk was used according to the protocol recommended by the Ministry of Agriculture, Livestock and Supply (MAPA). The milk samples were extracted with an organic solvent to obtain a purified extract. The sample was extracted with acetonitrile, passed through a cleanup step, evaporated, returned to the mobile phase, and the supernatant was analyzed directly in the LC-MS/MS system. Results are expressed as quantifiable residue levels. The analytes tested belong to the beta-lactam classes (penicillin G; penicillin V; ampicillin; amoxicillin; oxacillin; cloxacillin; dicloxacillin; ceftiofur; trimethoprim).
3. RESULTS AND DISCUSSION
In the samples qualitatively analyzed for the presence or absence of antibiotic residues, a percentage of 95.2% (40/42) of the milk samples did not detect the presence of antibiotic residues, and 4.8% (2/42) detected the presence of non-quantifiable levels by confirmatory analysis, where these levels are above the detection limit and below the quantification limit, thus falling within the standards established by legislation for the presence of antibiotic residues in milk.
The levels that cannot be quantified by confirmatory analysis represent the analytical performance (measurement uncertainty) at a given confidence level, detecting the sensitivity to Maximum Residue Limits (MRLs), as well as decision limit values (CCa) and detection capacity (CCβ), so CCa is the lowest concentration level at which the method can discriminate the presence of a compound with 95% statistical certainty (Brasil, 2006).
The Codex Alimentarius considers drug residues as the fraction of the drug and its biotransformed derivatives present in foods of animal origin that have been treated, and establishes that milk must be free of contaminants at levels that are harmful to the consumer population. Therefore, the legislation recommends the detection of antimicrobials in tank milk, respecting the MRLs for each specific group (Brasil, 2018).
During the survey, 100% (100/100) of dairy farmers reported that they had no cases of mastitis in their herd during the survey period and that they had not used any type of antibiotic treatment for any disease. However, when asked what type of antibiotic they used when cases of mastitis and other diseases occurred in the herd, 3% (3/100) of producers said they used tetracycline-based antibiotics, 2% (2/100) used fluoroquinolones, while 9% (9/100) used beta-lactams. The remainder reported that there were no diseases of any kind in the flock (25%), and the majority could not recall the type of medication used when necessary (61%).
It is worth noting that, according to the study by Silva, Silva and Ribeiro (2012), the most commonly used antimicrobials in the articles analyzed in their systematic review are tetracyclines (17.24%) and beta-lactams (13.79%).
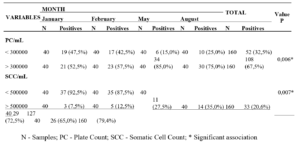
Although no antibiotic treatment was performed according to the manufacturer, the fluoroquinolone (norfloxacin) and tetracycline (oxytetracycline) classes were detected in these samples. Although detected, these drugs were not quantified as described in Table 01.
Table 01: Search for antibiotic residues in fresh milk in the state of Alagoas
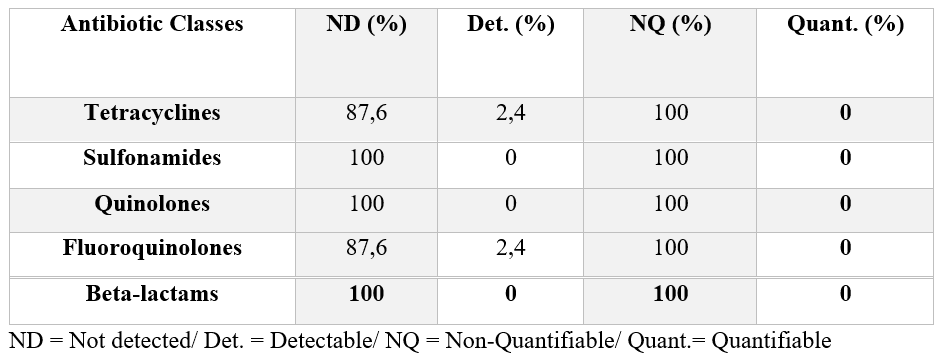
In this way, all the samples analyzed comply with Normative Instruction 76, which recommends the absence of antibiotic residues in cows’ milk. (Brasil, 2018).
According to the producers, the dairies that buy the milk control the quality of the product by testing for antibiotic residues, which they do in-house.
Cansan, Gorodicht and Kindlein (2023) found the presence of antimicrobial residues in 38.05% (242/636) of raw milk samples from dairies in Rio Grande do Sul, of which (394/636) were negative, representing 61.95%, with beta-lactams identified in 39.67%, followed by aminoglycosides in 25.21%.
No residue of antibiotics belonging to the beta-lactam class was detected in this study, however, the literature shows that beta-lactams are the antibiotics frequently used to treat mastitis in dairy cattle (38.22%), followed by tetracyclines with 15.41% (Sachi, 2019).
Alves et al. (2023) tested for antibiotic residues in refrigerated raw milk from 18 farms in Vitória da Conquista – BA, and found residues of antibiotics from the beta-lactam and tetracycline classes in two samples.
Carvalho et al. (2012), using the Delvotest – SP Kit, which uses microbial methodology (bacterial strains of Bacillus stearothermophilus), examined 18 properties in the rural area of the municipality of Araioses – MA during the rainy and dry seasons for the presence of residues and found 100% of the samples to be negative, as observed in this study.
Vieira et al. (2012) detected antimicrobial residues in 19% (15/79) of pasteurized milk samples from commercial establishments in the state of Paraná. They used commercial ELISA kits and found chloramphenicol, tetracyclines, gentamicin, streptomycin, and beta-lactams in the positive samples.
According to Poupaud et al. (2021), the reasons for controlling antibiotic residues in milk are due to the introduction of antimicrobial residues into the food chain, which contributes to antimicrobial resistance, causes economic problems for the dairy industry, contributes to human allergies due to the consumption of contaminated raw materials, since pasteurization is not able to inactivate the antibiotic residues present, thus compromising the food safety of the population.
4. CONSIDERATIONS
This study shows that there were no antibiotic residues at quantifiable levels in the samples analyzed. It is suggested that these antimicrobials be monitored in milk to ensure the absence of these residues and to guarantee a quality product, which is essential for public health.
REFERENCES
ALVES, C. C. et al. Qualidade físico-química e microbiológica de leite cru refrigerado na Região Sudoeste da Bahia. Rev. Saúde e Biol., v.18, e023004, 2023.
BRASIL, Ministério da Saúde (MS). Secretaria de Atenção à saúde. Coordenação Geral da Política de Alimentação e Nutrição. Guia Alimentar para população Brasileira: promovendo a alimentação saudável. Brasília: MS; 2006.
BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Instrução normativa nº 76, de 26 de novembro de 2018. Regulamentos Técnicos que fixam a identidade e as características de qualidade que devem apresentar o leite cru refrigerado, o leite pasteurizado e o leite pasteurizado tipo A. Diário Oficial da União, Brasília, 2018.
BROWN, K. Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera, Nairobi. PLOS ONE, v. 15, n. 5, p. 1–8, 2020.
CANSAN, I. C. S.; GORODICHT, M. A. M.; KINDLEIN, L. Análise dos resíduos de antimicrobianos no leite cru e avaliação dos riscos à saúde pública. Cuadernos de Educación y Desarrollo, v.15, n.4, p. 3211-3223.
CARVALHO, A. P. C. et al. Pesquisa de resíduos de antibióticos em amostras de leite cru no município de Araioses – MA. Revista Trópica: Ciências Agrárias e Biológicas V. 6, N.2, p. 22. 2012.
EL BEHIRY, A. et al. In vitro susceptibility of Staphylococcus aureus strains isolated from cows with subclinical mastitis to different antimicrobial agents. Journal of Veterinary Science. v.13, n.1, p.153-161. 2012.
POUPAUD, M. et al. Compreender a cadeia de suprimento de antibióticos veterinários para abordar a resistência antimicrobiana na RDP do Laos: Funções e interações das partes interessadas envolvidas. Acta Tropica, v. 220, p. 105943, 2021.
QUINTANILLA, P. Enrofloxacin treatment on dairy goats: Presence of antibiotic in milk and impact of residue on technological process and characteristics of mature cheese. Food Control, v. 123, p. 107762, 2021.
ROSA, A. A. et al. Estudo Comparativo da Qualidade Físico-Química e Microbiológica de Leite. PEER REVIEW, Vol. 5, Nº 9, 2023.
SACHI, S. Antibiotic residues in milk: Past, present, and future. Journal of advanced veterinary and animal research, v. 6, n. 3, p. 315, 2019.
SILVA, D. B. C. et al. Antibacterianos e condutas adotadas por produtores de leite em Goiás, Brasil. Cienc. Anim. Bras., V24, e-73715P, 2023.
SILVA, R. M.; SILVA, R. C.; RIBEIRO, A. B. Resíduos de Antibióticos em Leite. Rev. Saúde e Biologia, jan/abr, 2012.
VIEIRA, T. S. W. J. et al. Detecção de resíduos de antibióticos em amostras de leite pasteurizado do Estado do Paraná, Brasil. Semina: Ciências Agrárias, v. 33, n. 2, p. 791-796. 2012.
[1] PhD in Animal Bioscience. ORCID: https://orcid.org/0000-0003-2473-9451. Currículo Lattes: http://lattes.cnpq.br/5770903127454350.
[2] PhD in Animal Bioscience. ORCID: https://orcid.org/0000-0001-5078-4104. Currículo Lattes: https://lattes.cnpq.br/2775935070259137.
[3] PhD in Agricultural Biotechnology. ORCID: https://orcid.org/0000-0002-7654-5475. Currículo Lattes: http://lattes.cnpq.br/2574390886279350.
[4] PhD in Veterinary Medicine. ORCID: https://orcid.org/0000-0002-5917-5783. Currículo Lattes: http://lattes.cnpq.br/8326410355923632.
[5] PhD in Veterinary Medicine. ORCID: https://orcid.org/0000-0001-5565-507X. Currículo Lattes: http://lattes.cnpq.br/8867767311086224.
[6] Master in Animal Science. ORCID: https://orcid.org/0000-0002-7067-3855. Currículo Lattes: https://lattes.cnpq.br/7795984886994762.
[7] PhD in Agronomy (Soil Sciences). ORCID: https://orcid.org/0000-0002-0488-9236. Currículo Lattes: http://lattes.cnpq.br/4666659327763907.
[8] Master in Animal Science. ORCID: https://orcid.org/0000-0001-9152-9409. Currículo Lattes: http://lattes.cnpq.br/2231547122729508.
[9] Graduating in Bachelor of Biological Sciences. ORCID: 0000-0001-7149-4931. Currículo Lattes: https://lattes.cnpq.br/2928291078391850.
[10] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: 0000-0003-1493-7964. Currículo Lattes: http://lattes.cnpq.br/9044747136928972.
[11] PhD in Tropical Animal Science. ORCID: 0000-0001-5405-028X. Currículo Lattes: http://lattes.cnpq.br/8207812492517198.
[12] Advisor. PhD in the Postgraduate Program in Animal Bioscience. ORCID: 0000-0002-1289-2902. Currículo Lattes: http://lattes.cnpq.br/5998863169551704.
Submitted: September 1, 2023.
Approved: October 16, 2023.