ORIGINAL ARTICLE
BORGONOVI, Steven de Andrade [1], IBARRA, Gabrielle Amarilha [2], NECO, Gabriel Conforti Papa [3], RODRIGUES, Rayane Vieira [4], BARBOSA, Hueberton [5], PÁDUA, Aryston Vinicius Queiroz de Almeida [6], SANTOS, Alexsander Saves dos [7]
BORGONOVI, Steven de Andrade. Et al. Filtration Prototype Applied In Water Deionization Using Mixed Resin. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 06, Ed. 05, Vol. 14, pp. 61-72. May 2021. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/chemical-engineering/water-deionization
ABSTRACT
In this work, a prototype deionizer filter was designed, built and tested for crude water demineralization. The prototype has acrylic main body, with steps of coal, sand, stone and cationic and anionic resins, with a wooden base and a container for collecting deionized water. The crude water test was performed, with conductivity of 144,8.10-6 µs/cm before filtration, and then 20,15.10-6 µs/cm, then showing a considerable system efficiency. The prototype presented satisfactory results and met its purpose of creation, based on SANEPAR (2013), where for solutions with intermediate conductivity such as mineralized water, drinking water and wastewater, the range is 10.10-6 µs/cm by 2000.10-6 µs/cm.
Keywords: Low cost, Filtration, Deionizer, Water.
1. INTRODUCTION
According to Teruya (2012), filtration is an experimental technique of dividing heterogeneous mixtures composed of a solid state and a fluid state, as a rule, a liquid. It is widely used not only to extinguish solid particles of the fluid state, but also to secrete a dispersed solid or retained in a liquid. The filtration process can be used, not only on a laboratory scale, but also on an industrial scale.
Such filtration techniques are carried out: simple filtration, also known as common and vacuum filtration.
In the words of Salvador and Usberco (2006), in the simple filtration process, carried out in the laboratory, a circular filter paper is used, which is folded in half twice, so as to be divided into four parts. This paper increases the filtration surface area by speeding up the procedure. If the most relevant part is the solid residue that is retained in the filter paper, there is the possibility of even more folding it. This filter paper is then placed in an analytical funnel and with the aid of a glass drumdrum, the mixture is transferred. While solid particles are retained in the filter, the liquid passing through the pores is collected in another container. In the vacuum filtration process, the difference lies in the application of the vacuum (low pressure) inside the container, which will collect the filtered solution. In this process, due to the application of the vacuum, the occurrence of suction speeds up the procedure.
Among the filtration processes it is also possible to apply a filter for demineralization or deionization obtaining pure water. The water demineralizing process consists of the removal of ions (anions and cations) present in it, so the procedure is commonly called deionization. It has the same benefits and aspects of distilled water, however they are obtained by other procedures. This process happens with the permanence of an ion exchange resin composed of synthetic products, attached at the end of the filter, which allows the purification of water at the chemical level, with transition of contaminating ions by inert ions to the solution.
When placed in water, ion exchange resins may release sodium or hydrogen ions (cationic resins) or hydroxyl (anionic resins) and capture from this same water, respectively, cations and anions, responsible for their content of dissolved solids, undesirable to many industrial processes (SAKAI, 2012).
The most important change from demineralized water to distillate is that the first purification procedure does not make use of energy. Only specific resins are used that perform an ion exchange, thus emerging purified water. In addition to this there is also the procedure through reverse osmosis, in which filtration does not make use of chemicals and it is a mechanical process (AFONSO, 2015).
The most important is the final quality of the demineralized water produced and where it will be used. In the case of power plants, water will be widely used in boiler washes, while in laboratories, it applies for final washing of glasstiles.
The objective of this work was to build a deionizer filter using mixed resin and other filters and evaluate its operation for water filtration for industrial and laboratory consumption.
2. MATERIAL AND METHODS
The present project was carried out from August to December 2019 at University Brasil, located at Estrada Projeto F-1 s/n Fazenda Santa Rita, in the municipality of Fernandópolis-SP.
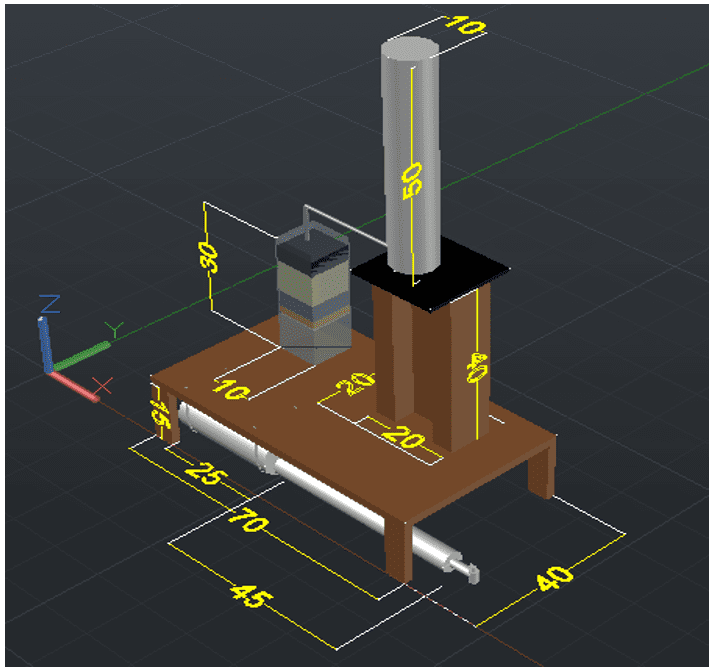
The first step was the choice and technical design of the equipment model according to Figure 1 with scale in mm, taking into account the best production and yield in filtration. The final product was set at 30cm for column length and width of 10cm.
Figure 1 – Technical design of the project.
Then, the materials listed in Table 1 were acquired. Some materials such as the sieve mesh, pipes and wooden platform were donated by Usina Bpbunge in the city of Ouroeste/SP and Madeireira Lourenção located in the city of Fernandópolis/SP. The aim was to use low-cost and reusable materials.
Table 1 – Materials and values.
| materials | quantity | Unit Value (R$) | Total Value (R$) |
| Acrylic filter 10×30 cm | 1 | 65,00 | 65,00 |
| Tap ¾ | 2 | 4,50 | 9,00 |
| Wooden tablet 70×40 cm | 1 | 0,0 | 0,0 |
| Rubber hose ¾ | 1m | 2,70 | 2,70 |
| Rubber hose ½ | 1m | 2,30 | 2,30 |
| PVC pipe 100mm (reservoir) | 20cm | 8,50 | 8,50 |
| PVC pipe 100mm | 30cm | 10,00 | 10,00 |
| Pvc pipe 50mm | 45cm | 8,00 | 8,00 |
| ¾ U clamp | 2 | 0,55 | 1,10 |
| ½ U clamp | 2 | 0,55 | 1,10 |
| saw | 1 | 0,0 | 0,0 |
| screw | 8 | 0,10 | 0,80 |
| Wooden platform 70x40cm | 1 | 0 | 0 |
| stylus | 1 | 0,0 | 0,0 |
| Silicone glue | 1 | 15,00 | 15,00 |
| Homemade wood holder 40x20cm | 1 | 0,00 | 0,00 |
| PVC cap 100mm | 4 | 2,00 | 8,00 |
| Sieve mesh 10x10cm | 1 | 0,0 | 0,0 |
| stone | 300g | 2,00 | 2,00 |
| sand | 500g | 3,00 | 3,00 |
| Pvc pipe 50mm | 1 | 8,00 | 8,00 |
| Activated carbon | 200g | 35,00 | 35,00 |
| Ionic resin | 500g | 60,80 | 60,80 |
| Total | – | – | 240,30 |
Source: The authors.
The next step was to the assembly of the external equipment with the wooden platforms, then two perforations of 13mm were carried out, at the top (inlet of raw water) and at the bottom (deionized water outlet), in the acrylic filter that has a measure of 10x10x30cm, with a volume of 3L, as shown in Figure 2.
Figure 2 – Assembly outside.

Next, the internal part of the filter was mounted, where the filtration process was carried out in order to remove all the ores and salts contained in the raw water, shown in Figure 3.
Figure 3 – Assembly inside.

The internal assembly step follows the sequence of a usual filter, starting with activated carbon that is one of the most important adsorbents, from the industrial point of view, being used for separation and purification of mixtures in gaseous and liquid phase, then the sand and gravel filters remove turbidity, particulates and small amount of emulsified material in colloidal form or emulsion , improving color and flavor, the mixed resin was subsequently introduced, which is composed of 50% cationic resin and 50% anionic resin, which is developed especially for water treatment in industries and laboratories, are used for removal of ions from water, after including a layer of cotton that helps in the removal of solids from water , due to the high contact surface, consequence of the micrometric dimensions of the wires and finished with a filter mesh to ensure a high efficiency of the filtration process.
After the process, the deionized water is destined for the next container, being conducted in rubber pipe, gravitationally, being received in a pipe reservoir with a volume of 2.45L, thus making the product stock, being represented by Figure 4.
Figure 4 – Storage process.

3. RESULTS AND DISCUSSION
Conductivity analyses were performed before and after each filtration. The results were tabulated and presented in Table 2:
Table 2 – Conductivity values.
| tests | Conductivity of raw water | Conductivity of deionized water |
| 1 | 144.8 | 20,15 |
| 2 | 164.3 | 17,56 |
| 3 | 164.3 | 16,21 |
| 4 | 164,3 | 16,14 |
| 5 | 166.1 | 17,25 |
Source: The authors.
There is a reduction in the conductivity rate equivalent to 70%. The ion exchange process took place, due to the use of synthetic resins, where they retain the salts dissolved in water through a chemical reaction, releasing equivalent ions to the solution. The raw water to be demineralized must be subjected to a pre-filtration for removal of suspended solids: clay, sand and others, including chlorine, which is removed at the beginning of filtration due to the presence of the atited coal. According to Saves (2018, apud SANTOS, 2019)
It is natural to saturation of the resin according to the time of use, so it is necessary that the produced water is frequently monitored with the aid of a conduitvimeter, allowing to determine when it should be replaced. The maximum level of conductivity that will determine when the resin should be changed will depend on the use for which purified water is intended, being established by the user for each application. Reference values are shown in Chart 1.
Table 1 – Conductivity reference values.
| application | belt |
| For solutions with low conductivity such as distilled, deionized or ultra-
Pure. |
0.5 μs until 400 μs |
| For intermediate conductivity solutions such as mineralized water, drinking water and water
Residual. |
10 μs until 2000 μs |
| For solutions with good conductivity such that seawater, acids, bases and diluted salts,
physiological solutions. |
1000 μs until 200,000 μs |
Source: SANEPAR, 2013.
The filter flow rate was calculated using Equation 1:
Equation 1 : 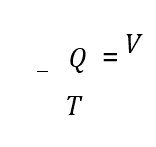
where Q is the flow, V is the volume, and t is the time.
The filter flow was performed, in which t=49.15s and V=0,003m³ and Q=6,1×10-5 m3/s was obtained. The volume occupied in the filter was calculated using Equation 2:
Equation 2: V = A.h
Where V is the prefilled filter volume, A is the filter area and h is the height occupied by the filter components. The prefilled volume was calculated with A= 0.01m² and h= 0.019m.
V = 0.00195 m3.
Table 3 – Calculation Result
| yield | ≅ 72% |
| Volume of the material contained in the filter | 0.00195 m3 |
| Filter volume | 0.003 m³ |
| Volumetric flow | 6.1×10-5 m3/s. |
Source: The authors.
4. CONCLUSIONS
It was concluded that the performance of the prototype was satisfactory when compared to the results published by SANEPAR (2013). The variation of the values obtained at the end of each sample depends on the water used in the filtration, however, the results obtained were linear during all tests, thus showing the efficiency of the system, since the process can be performed several times without the need to replacement the materials.
It is assumed that it is possible to increase the efficiency of the prototype, if changed the extension of the resin column, provided that the flow rate is maintained. The amount of resin/retention time, are crucial factors for prototype performance.
In a possible replication of the filter, decrease the empty space left at the end of the column, because it serves only to avoid reverse pressure.
REFERENCES
AFONSO, Julio Carlos. Separação sólido-líquido: Centrífugas e papeis filtro. Química Nova, volume nº38, no.05. São Paulo, Junho de 2015
SAKAI, Suzana. Resinas trocadoras de Íons, soluções a favor do tratamento de água e efluentes. Revista TAE, São Paulo, edição Nº 9 – outubro/novembro de 2012 – Ano 2.
SALVADOR, Edgard e USBERCO, João. Química, volume único. 1ª edição. São Paulo-SP: Editora Saraiva, 2006. 672 p.
SANEPAR. Companhia de Saneamento do Paraná, 2013. Disponível em: <https://site.sanepar.com.br/>
TERUYA, Leila Cardoso. Filtração Simples. LABIQ – Laboratório de Química e Bioquímica. Disponível em: <http://labiq.iq.usp.br/paginas_view.php?idPagina=4&idTopico=68#.YIs6H7VKjDc> Acesso em: 12 de outubro 2019.
[1] Academic in Chemical Engineering.
[2] Academic in Chemical Engineering.
[3] Academic in Chemical Engineering.
[4] Academic in Chemical Engineering.
[5] Academic in Chemical Engineering.
[6] Academic in Chemical Engineering.
[7] Advisor. Master’s degree in Environmental Sciences.
Submitted: February, 2021.
Approved: May, 2021.