ORIGINAL ARTICLE
CAVALCANTE, Luzia Angélica Alves [1], ANDRADE, Jéssica Martins de [2], SILVA, Maria Goretti Varejão da [3], MEDEIROS, Anna Karolyne de Araujo [4], CORDEIRO, Geovania de Souza [5], SILVA, Daniel Dias da [6], SOARES, Karla Danielle Almeida [7], MOURA, Vilton Edson Figueiroa de [8], SOARES, Anísio Francisco [9], MEDEIROS, Elizabeth Sampaio de [10]
CAVALCANTE, Luzia Angélica Alves. et al. Pseudomonas aeruginosa biofilm formers in drinking water. Revista Científica Multidisciplinar Núcleo do Conhecimento. Year 08, Ed. 09, Vol. 01, pp. 17-29. September 2023. ISSN: 2448-0959, Access link: https://www.nucleodoconhecimento.com.br/veterinaria-en/pseudomonas-aeruginosa, DOI: 10.32749/nucleodoconhecimento.com.br/veterinaria-en/pseudomonas-aeruginosa
ABSTRACT
Currently there has been an increase in demand for bottled water, this is due to the population’s dissatisfaction with the quality of water provided by public agencies. The present study sought to evaluate the presence of biofilm-forming P. aeruginosa in bottled drinking water. Thirty-five typical bottled water samples were selected to identify the presence of P. aeruginosa and its ability to form bacterial biofilm. The methodology used to verify the presence of P. aeruginosa followed the multiple tube technique and the evaluation of biofilm forming capacity followed the microdilution technique, with the optical capacity read by spectrophotometry at 620 nm. The presence of P. aeruginosa was detected in 26% of the samples analyzed, representing nine of the 35 samples tested. Of these, 55% had the ability to form biofilm. The present study shows that the presence of P. aeruginosa, which is capable of forming biofilms, is a risk factor for public health because of the widespread commercialisation of bottled water.
Keywords: Bacterial adhesion, Microbiological techniques, Packing industry.
INTRODUCTION
In recent years, Brazil has seen a steady increase in the demand for bottled water due to the lack of capacity of public supply systems to provide water of adequate quality and quantity (BRANDÃO et al., 2012). Although this product associates with its image a condition of purity and sanitary safety, the occurrence of gastrointestinal outbreaks due to the consumption of these waters has directed the attention of researchers to its study, especially with regard to its microbiological standard (COSTA et al., 2015; WIELAND et al., 2018).
Novello et al., (2020) state that rapid microbial growth after packaging may be related to oxygenation of the water, temperature increase during storage, and nutrients present on the packaging surface. It has been observed that this product is not necessarily safe and may pose health risks to consumers, such as aggravation by opportunistic pathogens such as Pseudomonas aeruginosa. This bacterium, due to its high resistance to antimicrobial therapy, is associated with persistent chronic infections, mainly affecting immunocompromised individuals (REIS et al., 2014; NOVELLO et al., 2020).
Contamination of these waters with P. aeruginosa during the filling process may be due to the ability of this bacterium to form biofilms on returnable packaging and plant equipment, and this property is considered one of the major virulence factors attributed to P. aeruginosa (PEDROSA et al., 2014).
The wide distribution and consumption of these waters among the population makes the monitoring of this product relevant. P. aeruginosa is one of the microbiological criteria defined in the current legislation for bottled water, but there is no requirement to investigate the ability of this microorganism to form biofilms. This research would have a significant impact on bottled water in particular, as the bottling process is conducive to the development of these biological structures. Therefore, recognizing the need for this research, the present study aimed to evaluate the occurrence of biofilm-forming P. aeruginosa in bottled drinking water.
METHODOLOGY
THE SAMPLES
Between the months of November 2019 and September 2020, 35 samples of non-carbonated bottled water marketed in 20-liter returnable bottles of 21 different brands (coded by the letters “A” to “U”) were separated and analyzed. Each representative sample was made up of 5 units of the same lot/bottle date, in order to comply with the sampling parameters of RDC ANVISA nº 275 of 22.09.2005 (Brasil, 2005), still in force during the period of analysis of this study, for a total 175 bottles. Nine samples of brand “D”, three of brands “H and I”, two of brands “B and C” and one of brands “A, E, F, G, J, K, L, M, N, O, P, Q, R, S, T and U” were analyzed. The samples came from the “Bottled Water Monitoring Program” of the Agência Pernambucana de Vigilância Sanitária (APEVISA) and the Laboratório Central de Pernambuco (LACEN-PE). The samples were selected according to the quantity received from the “Bottle Water Monitoring Program” of APEVISA and LACEN.
The research was developed in two phases: Research on P. aeruginosa in bottled water samples at the Laboratório de Microbiologia de Água e Alimentos (Coordenação de Vigilância Laboratorial em Bromatologia-LACEN/PE); Analysis of biofilm formation by P. aeruginosa isolated from bottled water samples at the Laboratório de Inspeção de Produtos de Origem Animal (Departamento de Medicina Veterinária/Universidade Federal Rural de Pernambuco (UFRPE).
DETERMINATION OF PSEUDOMONAS AERUGINOSA
Testing for P. aeruginosa in water samples was performed using the multiple tube technique recommended by the American Public Health Association (APHA) (APHA, 2017), which includes a presumptive and confirmatory phase. The sample was first prepared by homogenizing it by shaking the flask 25 times at an angle of 45°. In the presumptive phase, sterile asparagine broth was used in single and double concentrations with three dilution series (base 10), each series consisting of five tubes. After incubation at 36°C for 48 hours, a green fluorescent pigment in the specimen tube examined under long-wave ultraviolet light (365 nm) in a dark room was considered a positive presumptive test. The confirmatory phase was performed by inoculating the culture from the positive first phase tubes into sterile acetamide broth. The test is based on the ability of the bacteria to produce ammonia and acetic acid from acetamide and the change in pH of the medium, with a positive result for P. aeruginosa indicated by a change from orange to purple (presence of alkaline pH) within 4 to 7 days of incubation at 36°C.
- aeruginosa ATCC 27853 and Escherichia coli ATCC 25922 were used as positive and negative controls, respectively, in both phases. The number of positive tubes was calculated and analyzed using the most probable number (MPN) table with five tubes per dilution (10 mL, 1.0 mL, and 0.1 mL) (APHA, 2017).
ISOLATION OF PSEUDOMONAS AERUGINOSA
The acetamide broth tubes showing purple color were streaked on cetrimide agar by the depletion technique and incubated at 35 ± 2°C for 24 hours. One isolated green pigment-producing colony was selected from each sample. Colonies were seeded on nutrient agar to be collected for analysis of biofilm production capacity.
ANALYSIS OF BIOFILM PRODUCTION
To assess biofilm production, the methodology of Andrade (2018) was used with adaptations, where isolated P. aeruginosa colonies were inoculated into tubes containing 3 mL of BHI broth to McFarland’s 0.5 turbidity and incubated in a bacteriological oven at 37°C for 24 hours. After this step, 100 μL of the solution was inoculated with an automated micropipette into 96-well microdilution plates and incubated at 37°C for 24 hours. After this time, the contents of each well were gently aspirated with an automated micropipette and washed three times with sterile distilled water. Plates were allowed to dry at room temperature, and adherent cells were stained with 200 μL of 0.25% gentian violet. After 5 minutes of dye application, the wells were washed and dried at room temperature as previously described. Then 200 µL of 80:20 alcohol:acetone was added. BHI broth was used as a negative control and P. aeruginosa ATCC® 27853 was used as a positive control in triplicate. The optical density (OD) of the wells was read by spectrophotometry at 620 nm. To classify the isolates as to biofilm production, the mean optical density (OD) of the negative control (ODNC) was measured and compared with the mean OD of the isolates (ODIS), and they were classified as: negative (ODIS< ODNC); weak (ODNC< ODIS<2.ODNC); moderate (2.ODNC< ODIS< 4.ODNC); strong (4.ODNC< ODIS) biofilm formers.
DATA ANALYSIS
Descriptive statistical analysis was used to determine the absolute and relative frequencies of positive specimens relative to the total number of specimens collected. (SAMPAIO, 1998).
RESULTS AND DISCUSSION
Of 35 representative bottled water samples analyzed, 24 samples (69%) were typified as natural mineral water (NMA) and 11 samples (31%) as water with added salts (WAS).
Table 1. Pernambuco’s mesoregions of origin of the samples with respective amounts.
| Mesoregions | Quantity |
| MRR
Agreste Sertão Zona da Mata |
29
2 2 1 |
Source: Data obtained from the research conducted by the authors, 2023.
Table 1 shows the Pernambuco mesoregions of origin of the processed samples with the corresponding quantity. It can be observed that most of the samples came from the Metropolitan Region of Recife (MRR). The predominance of sampling in this mesoregion may be related to the concentration of bottling plants. Moreover, due to their geographical proximity, the samples from these bottlers reach consumers throughout the MRR, the most populated mesoregion of Pernambuco (IBGE, 2020), a relevant factor for health surveillance monitoring purposes.
Figure 1. Percent distribution of satisfactory (SAT) and unsatisfactory (INSAT) samples with respect to P. aeruginosa, according to the RDC nº 275/05 – ANVISA/MS
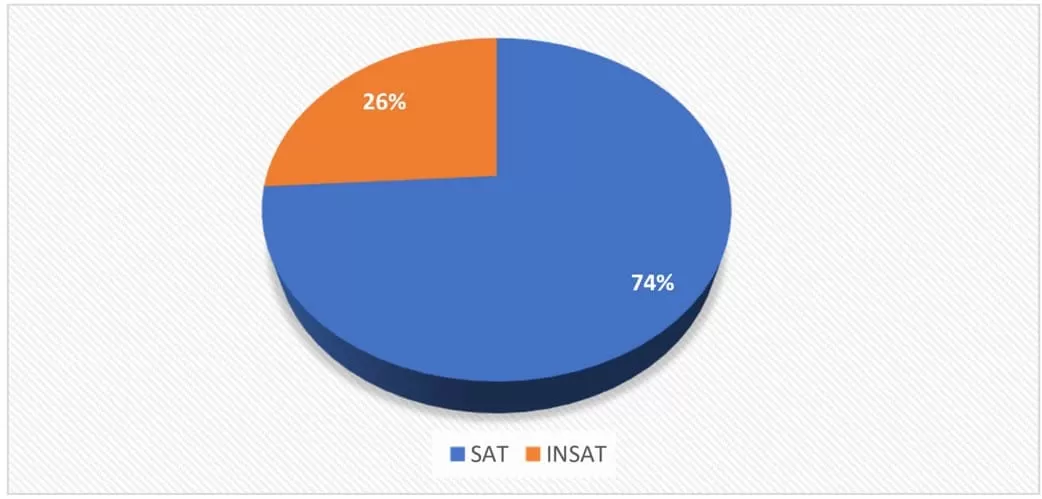
Figure 1 shows that 26% (9/35) of the samples analyzed were unsatisfactory for P. aeruginosa, according to the approval/rejection criteria defined by RDC ANVISA nº 275/2005 (BRASIL, 2005). This resolution specifies that the lot is rejected if it contains a count of this bacterium in any of the units of the representative sample, is higher than 2.2 MPN/100 mL or when the count of P. aeruginosa in more than one unit of the representative sample is higher than 1.1 MPN/100 mL.
Considering the unsatisfactory samples, Table 2 presents their P. aeruginosa levels (MPN/100mL) per sample unit. It is noteworthy that all samples in which the presence of this microorganism was verified were of sufficient quantity to reject the samples according to the microbiological standard for bottled water. It was also shown that among the unsatisfactory samples for the P. aeruginosa parameter, five were classified as natural mineral water and four as water with added salts. The presence of P. aeruginosa in water with added salts in quantities that make it unfit for consumption indicates the importance of including other microbiological parameters for this type of bottled water, in addition to those defined in the research period by RDC ANVISA Nº182/2017 (BRASIL, 2017). However, Pernambuco is in a position of reference in relation to this national resolution, as it has its own legislation for water with added salts, State Law Nº 15.859/2016 (PERNAMBUCO, 2016), which adopts as microbiological standard for this product the same one defined in the RDC ANVISA nº 275/2005 (BRASIL, 2005).
Table 2. Quantities of P. aeruginosa detected in bottled water samples by the most probable number technique (MPN/100 mL), per analytical unit
| Sample (brand/number) | Analytical Unit | Quantitative (MPN/ 100mL) |
| B2 | 1 | 41 |
| 2 | 79 | |
| 3 | < 1,8 | |
| 4 | < 1,8 | |
| 5 | < 1,8 | |
| D5 | 1 | 22 |
| 2 | < 1,8 | |
| 3 | < 1,8 | |
| 4 | 920 | |
| 5 | 26 | |
| D9 | 1 | < 1,8 |
| 2 | < 1,8 | |
| 3 | 21 | |
| 4 | < 1,8 | |
| 5 | < 1,8 | |
| J14 | 1 | 2 |
| 2 | < 1,8 | |
| 3 | < 1,8 | |
| 4 | 4,5 | |
| 5 | < 1,8 | |
| D16 | 1 | 130 |
| 2 | 350 | |
| 3 | 350 | |
| 4 | 220 | |
| 5 | 920 | |
| L17 | 1 | < 1,8 |
| 2 | 6,1 | |
| 3 | < 1,8 | |
| 4 | 4,5 | |
| 5 | < 1,8 | |
| D20 | 1 | 34 |
| 2 | 1600 | |
| 3 | 34 | |
| 4 | 21 | |
| 5 | < 1,8 | |
| I23 | 1 | < 1,8 |
| 2 | 2 | |
| 3 | < 1,8 | |
| 4 | 2 | |
| 5 | < 1,8 | |
| O27 | 1 | < 1,8 |
| 2 | 2 | |
| 3 | 2 | |
| 4 | < 1,8 | |
| 5 | < 1,8 |
Source: Data obtained from the research conducted by the authors, 2023.
Corroborating the findings of this study, a study by Brandão et al. (2012), of 31 samples of water bottled in 20-liter returnable bottles found that 17 samples had counts above the established limit for P. aeruginosa. Similarly, Coelho et al. (2010), when evaluating the microbiological quality of mineral waters consumed in the metropolitan region of Recife, found the presence ofPseudomonas spp. in 24 samples (20.00%), while P. aeruginosa was positive in 22 samples (18.33%), found in all ten brands analyzed.
In other countries, studies have also verified the positivity of this microorganism in bottled water. In a large study (2,500 samples) in Bulgaria, Georgieva and Dimitrova (2016) found this agent in 274 samples of bottled water analyzed, leading the authors to question the quality of this product for groups with compromised immunity. In a study by Herath (2014), 36 brands of bottled water from across the island of Sri Lanka were subjected to the determination of P. aeruginosa, and it was found that 18 (50%) brands showed the presence of this microorganism, and it is reported that these findings are due to improper UV treatment, clogged filters and cross-contamination, or the use of water from an inadequate source for bottling.
Kouchesfahani et al., (2015), while investigating P. aeruginosa in bottled water sold in Iranian markets, detected this microorganism in 36.7% of all samples tested. These authors note that, contrary to public opinion, bottled water is not free of microorganisms and suggest that authorities should provide a more rigorous monitoring and control plan.
In view of the results obtained in this study, there is congruence with the data already published by the aforementioned authors regarding the presence of this microorganism in significant quantities, and this occurrence is predominant when packaging is done in reusable containers, as well as agreement regarding the probable factors that lead to this contamination: failures in good manufacturing practices (GMP) during filling and storage, as well as inadequate washing and disinfection of reusable bottles, which facilitate the formation of biofilms by P. aeruginosa.
The production of biofilms is a characteristic of these bacteria and in this research we also tried to evaluate the isolates found in terms of their capacity to form biofilms (CFB) (Figure 2).
Figure 2: Percentage distribution of samples positive for P. aeruginosa according to their CFB
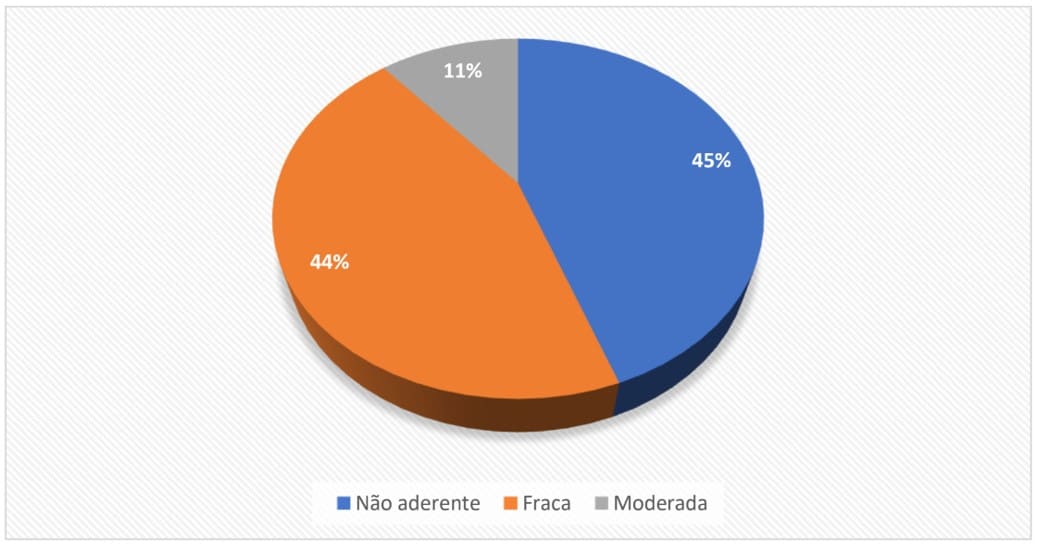
The collected results show that of the P. aeruginosa isolates tested for biofilm production, 55% were characterized as biofilm-forming and their CFB was rated between weak and moderate. Some authors also presented significant percentages of biofilm formation by P. aeruginosa from bottled water in their research. Pedrosa et al. (2014) found that out of 31 isolates of P. aeruginosa found in natural mineral water samples, 48% showed moderate to strong CFB and 51% were between nonadherent and weakly adherent.
Bernardo (2009) describes the classification of P. aeruginosa isolates from mineral water samples with respect to biofilm production, where 52.9% of the strains were classified as strongly adherent, 41.2% of the isolated strains as moderately adherent and 5.9% as weakly adherent.
When Rojas et al. (2014) evaluated CFB in P. aeruginosa strains isolated from bottled water samples, they found that they had a moderate ability to form biofilms. The importance of studying the biofilms in these isolates is evident, considering that they come from a product whose packaging tends to collaborate with the installation of these biological structures, especially when it comes to reusable packaging, which requires a strict hygienic standard that is highly susceptible to failure. Analyzing the results of this study and the others mentioned above on the occurrence of this bacterium in bottled water and its ability to form biofilms, we can see the relevance of laboratory surveillance actions on this product, as well as the existence of legal instruments that subsidize them. Current regulations set limits for P. aeruginosa in bottled water, but don’t include CFB testing of the isolates found. This inclusion would make it possible to evaluate the degree of pathogenicity of the P. aeruginosa found, which could be an aggravating factor in the health violation.
Considering that Pseudomonas aeruginosa are an opportunistic pathogen, which has a lot of resistance to antimicrobial groups (Pires et al, 2009), your occurrence represents a real risk to public health.
CONSIDERATIONS
The occurrence of P. aeruginosa in bottled water consumed in the State of Pernambuco, and the strains found were characterized as biofilm formers, is a virulence factor of great public health importance. In view of the clinical implications of this bacterium, the use of bottled water contaminated with P. aeruginosa endangers the health of consumers, especially those with compromised immune systems. The wide commercialization of these waters observed in recent years in the State of Pernambuco, added to the pathogenic potential of this bacterium, makes it preponderant continuous control actions on this product, aiming at a more efficient sanitary surveillance work in the bottling industries, which seeks to eradicate this microorganism and the consequent formation of its biofilm during the bottling process.
REFERENCES
APHA – AMERICAN PUBLIC HEALTH ASSOCIATION. Standard Methods for the Examination of Water and Wastewater. 23 ed., Washington, D.C.: APHA, 2017.
ANDRADE, J. M. Listeria monocytogenes formadoras de biofilme em presuntos fatiados e sua sensibilidade aos sanitizantes, 2018. Dissertação (Mestrado em Biociência Animal) – Universidade Federal Rural de Pernambuco, Recife, 2018.
BERNARDO, S. P. C. Avaliação da suscetibilidade a antimicrobianos e formação de biofilmes em Pseudomonas aeruginosa isoladas de água mineral, 2009. Dissertação (Mestrado em Vigilância Sanitária) – Instituto Nacional de Controle de Qualidade em Saúde, Fundação Oswaldo Cruz, Rio de Janeiro, 2009.
BRANDÃO, M. L. L. et al. Comparação das técnicas do número mais provável (NMP) e de filtração em membrana na avaliação da qualidade microbiológica de água mineral natural. Revista do Instituto Adolfo Lutz, São Paulo, v. 71, n. 1, p. 32-39, 2012.
BRASIL. Ministério da Saúde. Resolução – RDC nº 182, de 16 de outubro de 2017. Dispõe sobre as boas práticas para industrialização, distribuição e comercialização de água adicionada de sais. Diário Oficial [da] União, Brasília, DF, 16 out. 2017.
BRASIL. Ministério da Saúde. Resolução – RDC nº 275, de 22 de setembro de 2005. Aprova o regulamento técnico de características microbiológicas para água mineral natural e água natural. Diário Oficial [da] União, Brasília, DF, 22 set. 2005.
COELHO, M. I. S. et al. Avaliação da qualidade microbiológica de águas minerais consumidas na região metropolitana de Recife, Estado de Pernambuco. Acta Scientiarum Health Sciences, v. 32, n. 1, p. 1-8, 2010.
COSTA, D. et al. Nosocomial outbreak of Pseudomonas aeruginosa associated with a drinking water fountain. Journal of Hospital Infection, v. 91, n. 3, p. 271-274, 2015.
GEORGIEVA, V.; DIMITROVA, Y. Study of the Microbiological Quality of Bulgarian Bottled Water in Terms of Its Contamination with Pseudomonas aeruginosa. Central European journal of public health, v. 24, n. 4, p. 326-330, 2016.
HERATH, A. T. et al. Pseudomonas aeruginosa in bottled drinking water in Sri Lanka: a potential health hazard. Water Supply, v. 14, n. 6, p. 1045-1050, 2014.
INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA – IBGE. Estimativas da população residente para os municípios e para as unidades da federação brasileiros com data de referência em 1º de julho de 2020. Rio de Janeiro: IBGE, 2020. Disponível em: https://biblioteca.ibge.gov.br/index.php/bibliotecacatalogo?view=detalhes&id=2101747. Acesso em: 02 maio 2023.
KOUCHESFAHANI, M. M. et al. Pseudomonas aeruginosa and Heterotrophic Bacteria Count in Bottled Waters in Iran. Iranian Journal of Public Health, v. 44, n. 11, p. 1514-1519, 2015.
NOVELLO, J. et al. Inactivation of Pseudomonas aeruginosa in mineral water by DP1 bacteriophage immobilized on ethylene-vinyl acetate copolymer used as seal caps of plastic bottles. Journal of Applied Polymer Science, v. 137, n. 35, p. 1-8, 2020.
PEDROSA, A. P. et al. Pesquisa de fatores de virulência em Pseudomonas aeruginosa isoladas de águas minerais naturais. Revista Ambiente & Água, v. 9, n. 2, p. 313-324, 2014.
PERNAMBUCO. Lei Estadual nº 15.859, de 30 de junho de 2016. Dispõe sobre as condições sanitárias relativas à industrialização, distribuição e comercialização de água adicionada de sais no Estado de Pernambuco e dá outras providências. Recife: Assembleia Legislativa do Estado de Pernambuco, 2016.
PIRES, E. J. V. C. et al. Análise epidemiológica de isolados clínicos de Pseudomonas aeruginosa provenientes de hospital universitário. Rev Bras Ter Intensiva. v. 21, n. 4, p. 384-390, 2009. DOI: 10.1590/S0103-507X2009000400008.
REIS, L. R.; BEVILACQUA, P. D.; CARMO, R. F. Água envasada: qualidade microbiológica e percepção dos consumidores no município de Viçosa (MG). Cadernos saúde coletiva, v. 22, n. 3, p. 224-232, 2014.
ROJAS, T. et al. Bacilos gramnegativos no fermentadores en agua embotellada: susceptibilidad antimicrobiana y formación de biopelículas. Revista de la Sociedad Venezolana de Microbiologían, v. 34, n. 2, p. 64-69, 2014.
SAMPAIO, I. B. M. S. Estatística aplicada à experimentação animal. Belo Horizonte: FEPMVZ, 1998.
WIELAND, K.; CHHATWAL, P.; VONBERG, R. P. Nosocomial outbreaks caused by Acinetobacter baumannii and Pseudomonas aeruginosa: Results of a systematic review. American journal of infection control, v. 46, n. 6, p. 643-648, 2018.
[1] Master in Animal Bioscience. ORCID: https://orcid.org/0000-0002-0833-7201. Curriculum Lattes: http://lattes.cnpq.br/0965196379965915.
[2] PhD in Animal Bioscience. ORCID: 0000-0002-2871-6655. Curriculum Lattes: http://lattes.cnpq.br/3276511555193502.
[3] Doctoral student of the Graduate Program in Animal Bioscience. ORCID: 0000-0001-9410-7631. Curriculum Lattes: http://lattes.cnpq.br/4532231119888940.
[4] Graduate student in Veterinary Medicine. ORCID: 0000-0001-9273-5204. Curriculum Lattes: http://lattes.cnpq.br/8329028352662293.
[5] Graduate student in Veterinary Medicine. ORCID: 0000-0001-5584-9464. Curriculum Lattes: http://lattes.cnpq.br/1495170820726310.
[6] M.Sc. in Animal Bioscience. ORCID: 0000-0002-4913-8313. Curriculum Lattes: http://lattes.cnpq.br/4967459162060058.
[7] PhD in Animal Bioscience UFRPE. ORCID: https://orcid.org/0000-0003-2473-9451. Curriculum Lattes: http://lattes.cnpq.br/5770903127454350.
[8] Graduating in Bachelor of Biological Sciences. ORCID: 0000-0001-7149-4931. Lattes curriculum: https://lattes.cnpq.br/2928291078391850.
[9] PhD in Biochemistry and Physiology, Master in Physiology, Biologist. ORCID: 0000-0003-1493-7964. Curriculum Lattes: http://lattes.cnpq.br/9044747136928972.
[10] Advisor. Doctor of the Graduate Program in Animal Bioscience. ORCID: 0000-0002-1289-2902. Curriculum Lattes: http://lattes.cnpq.br/5998863169551704.
Submitted: July 3, 2023.
Approved: August 23, 2023.